- Laboratory >
- Laboratory medicine >
- Clinical test kit
Clinical test kits
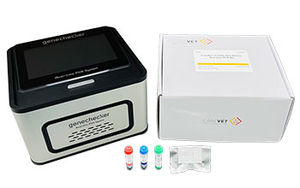
Result display time: 35 min
... IMPORTANCE OF SYSTEMATIC DETECTION OF FELINE URTD PATHOGENS Feline upper respiratory tract disease (URTD) is a common clinical problem in cats. The viruses and bacteria that cause URTD are highly contagious, so susceptible ...

Result display time: 15 min - 20 min
... instrument Contents: 1) Test card: It consists of reagent strip and plastic bag. 2) Sample diluent 3) Capillary tube 4) User manual Advantages: Be used directly at the point of care testing (POCT) Independent ...

... with all relevant clinical and laboratory finding. RTA HBV Real-Time PCR Kit has been validated for use with RTA Viral DNA Isolation Kit or RTA Viral Nucleic Acid Isolation Kit ...
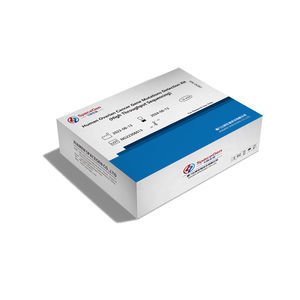
Result display time: 4 h
... system malignancies. Ovarian cancer is often referred to as the "silent killer" because when symptoms appear and patients seek medical attention, about 70% of cases are already in advanced stages, resulting in low 5-year ...
SPACEGEN
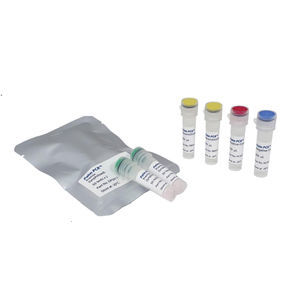
Result display time: 24 min
Specificity: 100 %
Sensitivity: 100 %
... Specific Test: Detect the SARS-CoV-2 specific RdRP gene with no cross-reactivity. ➢ Ultimate High Sensitivity: Detect down to 5 copies/reaction of SARS-CoV-2 (LoD: 5.3 copies/reaction). ➢ 100% Accuracy : 100% PPA ...
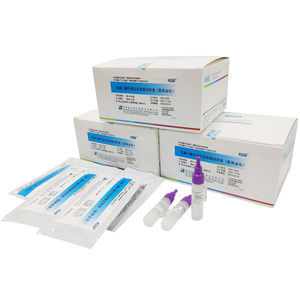
Helicobacter Pylori(H. Pylori)antigen Diagnostic Kit is an in vitro immunochromatographic assay for the qualitative detection of H.Pylori antigen in human faeces . It is related with cytotoxin released ...
Shenzhen kangshengbao Biotechnology Co., Ltd.

Result display time: 5 min - 10 min
Specificity: 94.7 %
Sensitivity: 97.8 %
... environments. Lateral flow tests are used for medical diagnostics and detect the presence or absence of a target analyte in a sample matrix without the need for additional specialized and expensive medical ...

... types of respiratory viruses and 6 types of pneumonia-causing bacteria Respiratory Pathogens Real-time PCR Kit is an in vitro diagnostic test for the qualitative detection of 19 respiratory ...

... cervical cancers in the end. PANA RealTyper™ HPV kit is an in vitro diagnostic reagent for genotyping of human papilloma virus (HPV) using peptide nucleic acid (PNA) probes. This kit ...

Result display time: 5 min - 20 min
Sample volume: 0.02, 0.01 ml
Specificity: 97.2, 98.6, 97.7 %
... plasma or whole blood. This test is for in vitro professional diagnostic use and intended as an aid to early diagnosis of HCV infection in patients with clinical symptoms of HCV infection. ...
SD BIOSENSOR. INC

Result display time: 10 min
... detect the presence of Anti-Golimumab antibodies with a small amount of whole blood and serum in 10 minutes. The test result can help with clinical decision making.

... conjunction with clinical presentation and the results of other laboratory tests. Results from the Amazing COVID-19 Ag/Ab(IgG/IgM) Sealing Tube Twin Test Strip should not be used as the ...
Amazing Biotech Co., Ltd

This test kit is used toclinical in vitrodetection ofMyoglobin (Mb)concentration inhuman blood quantitatively. 4. Clinical significance Myoglobin (Mb) is an oxygen-binding hemoglobin, ...

Result display time: 40 min
Specificity: 100 %
... internal standard is normal, to avoid false negative results. PACKAGING SPECIFICATIONS: 50tests/kit & 200tests/box STORAGE CONDITIONS AND EXPIRY DATE *The kit ...
HUNAN RUNMEI GENE TECHNOLOGY CO.,LTD
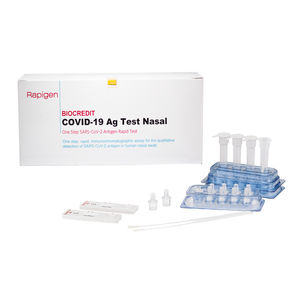
Result display time: 10 min - 15 min
Specificity: 100 %
Sensitivity: 98.1, 93.1 %
... patient with clinical symptoms. It provides only an initial screening test result and more specific alternative diagnosis methods should be performed in order to confirm COVID-19 infection. Principle ...
Biz Distribution

Result display time: 15 min
The Influenza A+B Test detects and differentiates influenza type A and type B antigens directly from nasal swab and nasopharyngeal swab specimens. A single sample can be used to run both the Influenza A+B Test ...

Result display time: 75 min - 80 min
Sensitivity: 96.4 % - 99.5 %
... Standard Clinical sensitivity: 99.51% Clinical specifity: 96.44% Overall coincidence: 97.49% Product Information Registration certificate: CE:Ref. No.:GZ 8821-2020; China NMPA: GUOXIEZHUZHUN ...

This test kit ensures the in vitro qualitative detection and differentiation of Canine Parvovirus (CPV) and Canine Coronavirus (CCV) in canine fecal samples. Details Box Content • Sample Collection ...
Your suggestions for improvement:
the best suppliers
Subscribe to our newsletter
Receive monthly updates on this section.
Please refer to our Privacy Policy for details on how MedicalExpo processes your personal data.
- Brand list
- Manufacturer account
- Buyer account
- Our services
- Newsletter subscription
- About VirtualExpo Group



















Please specify:
Help us improve:
remaining