- Laboratory >
- Laboratory medicine >
- Coronavirus test kit
Coronavirus test kits

Result display time: 120 min
... than 40 human infections with a novel coronavirus in an outbreak of pneumonia under investigation in Wuhan City, Hubei Province, China. The Chinese authorities identified a new type of coronavirus (novel ...

Result display time: 60 min
... - SLAN-96P, ABI7500, QuantStudio 5, S-Q31A, S-Q31B Sensitivity - 200copies/mL(Sample Release Reagent ); 50copies/mL(Nucleic Acid Extraction-Purification Kit ) Spec. - 24T/48T/96T
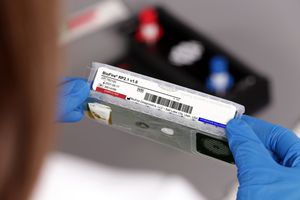
Result display time: 45 min
Specificity: 99.3 %
Sensitivity: 97.1 %
... high efficiency and throughput on the BioFire® FilmArray® 2.0 and the BioFire® FilmArray® Torch Systems. Rapid respiratory PCR test results may enable better-informed diagnosis and treatment of patients. Quick turnaround ...
BioFire Diagnostics

Bosphore SARS-CoV-2 / Flu / RSV Panel Kit is a Real-Time PCR based in-vitro diagnostic medical device, IVD CE marked according to 98/79/EC Directive. Bosphore SARS-CoV-2/Flu/RSV Panel Kit detects ...
Anatolia Tani ve Biyoteknoloji Urunleri Sanayi ve Ticaret A.S.
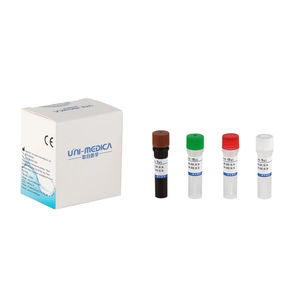
Result display time: 52 min
... real-time RT-PCR reagent kit to allow laboratories to diagnose the disease caused by 2019-nCov infection with fast and easy to use assays. Shenzhen Uni-medica RT PCR detection kit is ...
Shenzhen unimed-global technology Co.,LTD
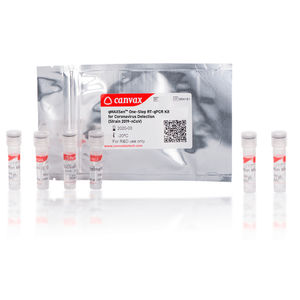
... washes or swabs; tracheal aspirates). qMAXSen™ Coronavirus (SARS-CoV-2) RT-qPCR Detection Kit [N and RNAse P genes-based Assay] allows efficient cDNA synthesis and Real-Time PCR in ...
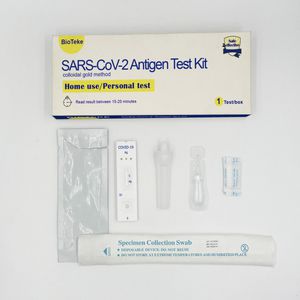
Result display time: 10 min - 20 min
Sensitivity: 90 % - 98 %
... saliva collectors Nasal sample collection - 1 kit /3 kit/ 5 kit /20 kit - test card ,sample duilent, sample extraction tube,sampling swab Nasopharyngeal ...
Bioteke Corporation
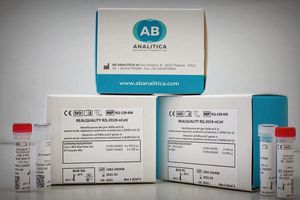
REALQUALITY RQ-2019-nCoV is a diagnostic CE-IVD kit for the identification of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) by one-step Real-Time RT-PCR. Peculiarity One step Real ...
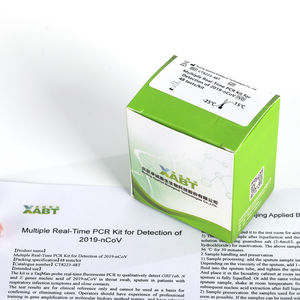
Multiple Real-Time PCR Kit for Detection of 2019-nCoV is a Real-Time RT-PCR test intended for the qualitative detection of nucleic acid from the 2019-nCoV in oropharyngeal swabs, nasopharyngeal swabs ...

... SARS-CoV-2 Detection Kit (Fluorescence RT-PCR) Sample Type: Respiratory specimen such as oropharyngeal (throat) swab, nasopharyngeal swab Target Gene: ORF1ab Gene, N Gene Analytical Sensitivity(LoD): ...
Result display time: 15 min
Specificity: 99.3 %
Sensitivity: 93.1 %
... extraction specimen from the extraction tube into the sample well of the test device by inverting and squeezing the tube as shown. Read the test results after 15 minutes. Do not read test ...
Jiangsu Konsung Medical Equipment
Result display time: 10 min
FLUORESCENCE IMMUNOCHROMATOGRAPHIC ASSAY AG TEST KIT Focused and dedicated to the rapid test, PCR and FIA technology development for the medical industry, Bioeasy offer the PCR, FIA ...

... MERS-CoV A real time molecular test for the screening and the differentication of Influenza A and B strains, COVID-19 and MERS-CoV Product Code:GRA2032 Detection: Flu A Flu B SARS-CoV-2 MERS-CoV Specimen: Nasopharyngeal ...
Medical Innovation Ventures

Result display time: 15 min
... Validity period :18 months Product specifications: 50 tests/box It is only used as a supplementary test indicator for suspected cases with a negative nucleic acid test of the new ...
Guangzhou Biotron Technology Co.,Ltd
Result display time: 15 min - 20 min
1 Test/Kit; 20 Tests/Kit INTENDED USE This kit is only used for the in vitro qualitative detection of 2019-nCoV antigen from human nasopharyngeal ...
hecin-scientific

The ImmTekTM COVID-19 product line aims to ease the burden of diagnosis on health facilities. We hope each product category can play a critical and complementary role in response to COVID-19 pandemic from early-stage detection to epidemiological ...

Result display time: 15 min
Specificity: 98.3 %
Sensitivity: 92.7 %
... by new coronavirus infections. It is not suitable for screening by the general population. A positive test result requires further confirmation, and a negative test result cannot ...

... - reaction with other coronavirus • Compatible with mainstream qPCR devices COVID-2019 Virus Nucleic Acid Detection Kit(Dual-RTPCR) 【Product Introduction】 This kit is used for ...

Result display time: 15 min
... -2 infection in direct saliva sample from suspected individuals of symptomatic or asymptomatic with COVID-19. Test Principle: This kit is a colloidal gold immunochromatographic testing product for ...
Changzhou Biowin Pharmaceutical Co., Ltd.

Result display time: 90 min
Sample volume: 0.2 ml
... COVID-19 Detection Kit directly detects SARS-CoV-2 genetic material. Main Features Qualitative detection of the novel coronavirus SARS-CoV-2 Real-time PCR methodology Targets specific ...
Convergent Technologies

Result display time: 15 min
... IgG Rapid Test (Lateral Flow Method) is an immunochromatographic assay for rapid, qualitative detection of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) IgM/IgG antibodies in human whole ...

Result display time: 20 min
Antigen Test detects the nucleoprotein of novel coronavirus in human nasopharyngeal(NP)or oropharyngeal(OP)swabs sample.COVID-19 antigen can detect the presence of viral protein. It can identify acute ...

Result display time: 6 min
... , and even life-threatening. It is very necessary to carry out an effective antibody covid test through the covid antibody test kit. 2019-nCoV Antibody Test Kit ...
Hipro Biotechnology Co., Ltd.
Result display time: 15 min - 20 min
Sample volume: 0.1 ml - 0.5 ml
... Detection Kit(Latex immunochromatography)is a lateral flow immunoassay intended for the qualitative detection of nucleoprotein from SARS-CoV-2 innasal swabs,pharyngeal swabs,sputum,bronchoalveolar,lavage fluid for Rapid ...
... ). SARS-CoV-2 (Severe Acute Respiratory Syndrome Coronavirus 2) is a new strain of coronavirus emerged from Wuhan, China in December 2019. The infection with SARS-CoV-2 virus causes Coronavirus ...
ADALTIS
Your suggestions for improvement:
the best suppliers
Subscribe to our newsletter
Receive monthly updates on this section.
Please refer to our Privacy Policy for details on how MedicalExpo processes your personal data.
- Brand list
- Manufacturer account
- Buyer account
- Our services
- Newsletter subscription
- About VirtualExpo Group



























Please specify:
Help us improve:
remaining