- Laboratory >
- Laboratory medicine >
- CTnT test kit
CTnT test kits
{{product.productLabel}} {{product.model}}
{{#if product.featureValues}}{{product.productPrice.formattedPrice}} {{#if product.productPrice.priceType === "PRICE_RANGE" }} - {{product.productPrice.formattedPriceMax}} {{/if}}
{{#each product.specData:i}}
{{name}}: {{value}}
{{#i!=(product.specData.length-1)}}
{{/end}}
{{/each}}
{{{product.idpText}}}
{{product.productLabel}} {{product.model}}
{{#if product.featureValues}}{{product.productPrice.formattedPrice}} {{#if product.productPrice.priceType === "PRICE_RANGE" }} - {{product.productPrice.formattedPriceMax}} {{/if}}
{{#each product.specData:i}}
{{name}}: {{value}}
{{#i!=(product.specData.length-1)}}
{{/end}}
{{/each}}
{{{product.idpText}}}
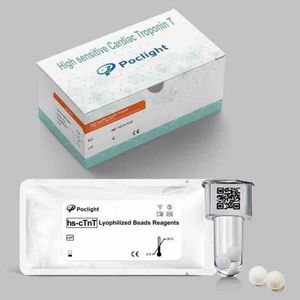
Result display time: 10 min
This assay is intended for in vitro the quantitative determination of Cardiac Troponin T in human serum and plasma.The test kit is used as an aid in the diagnosis of myocardial infarction. ...

... sensitive Cardiac Troponin T (hs-cTnT) test kit employs a wash-free and homogeneous strategy based on proximity hybridization-regulated CRET(Chemiluminescence resonance ...

hs-cTnI Detection Kit (CLIA) MYO Detection Kit (CLIA) CK-MB Detection Kit (CLIA) NT-pro-BNP Detection Kit ...

YHLO also supports a series of routine tests to meet different clinical needs, including Thyroid, Tumor Markers, Fertility, Anemia, Cardiac Markers, Liver Fibrosis, Metabolism, and Bone Metabolism. Immunoassay ELISA ...
Shenzhen Yhlo Biotech Co., Ltd.
It is mainly used in the auxiliary diagnosis of Myocardial Infarction in clinic.
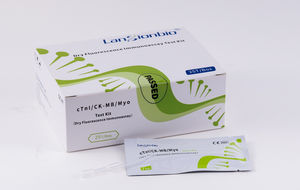
cTnl /CK-MB/Myo Test Kit (Dry Fluorescence Immunoassay) is intended for in-vitro quantitative measurement of cTnl /CK-MB/Myo (Cardiac Troponin 1/ Creatine Kinase Isozyme/Myoglobin ) in human serum . cTnl ...
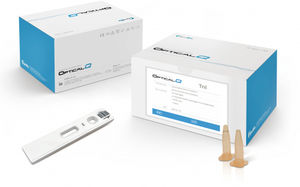
Result display time: 15 min
Sample volume: 0.1 ml
... muscle contraction in skeletal muscle and cardiac muscle. Troponin levels increase when heart muscles are damaged. Troponin tests thus measure the level of cardiac-specific troponin in the blood to help detect heart injury. ...

Result display time: 12 min
... ichroma™ (0.1 – 50 ng/ml) Indication Acute myocardial infarction, acute coronary syndrome In Your Medical Field This test can provide quick and accurate information to distinguish between myocardial infarction ...
Your suggestions for improvement:
the best suppliers
Subscribe to our newsletter
Receive monthly updates on this section.
Please refer to our Privacy Policy for details on how MedicalExpo processes your personal data.
- Brand list
- Manufacturer account
- Buyer account
- Our services
- Newsletter subscription
- About VirtualExpo Group










Please specify:
Help us improve:
remaining