- Laboratory >
- Laboratory medicine >
- Infectious disease test kit
Infectious disease test kits


Result display time: 30 min
Specificity: 100 %
Sensitivity: 98 %
... fatal in immunodepressed patients. Serum-negative female patients who become infected during pregnancy may transmit the disease to the fetus. In 95% of cases this occurs without symptoms, but some neonates may present ...
Diesse Diagnostica Senese

Regulatory Status (USA):510(k) Cleared Discipline:Infectious Disease
Gold Standard Diagnostics

... visualization of color change upon sample addition to microwells. Kit Configuration and Principle of Test The product is available in three kit configurations, the 384, 960, and 9600 ...
... Quantitative RT-PCR Kit is an in vitro diagnostic kit designed for the quantification of hepatitis C virus (HCV) RNA in human samples such as serum and EDTA-plasma through real-time PCR. Product Description Hepatitis ...
Bioneer Corporation
ELISA test for the qualitative determination of Escherichia coli O157 antigen in human stool specimens.

Bosphore HCV Quantification Kit, is a Real-Time PCR based in-vitro diagnostic medical device, IVD CE marked according to 98/79/EC Directive. Bosphore HCV Quantification Kit detects and quantitates hepatitis ...
Anatolia Tani ve Biyoteknoloji Urunleri Sanayi ve Ticaret A.S.
Result display time: 15 min
Sample volume: 0.02 ml
... gastrointestinal disease. The test is intended for use by health care professionals Principle of The Test The Rapid H.pylori Antibody Test Kit is ...

Result display time: 5, 10 min
Sample volume: 0.01 ml
The DRG Schistosoma IgG ELISA is for the qualitative determination of serum or plasma antibodies in humans, primarily IgG, to Schistosoma spp. using the ELISA technique. During the first incubation, ...
DRG Instruments GmbH

... B Detection Kit(Fluorescence PCR) is a qualitative in vitro real-time RT-PCR test for the detection of nucleic acid from influenza A virus or influenza B virus in specimens from individuals suspected ...
Healgen Scientific

... months. This test is a rapid (10 minute) assay based on the detection of adult female Dirofilaria antigen present in the dog’s serum, plasma or whole blood. The assay uses sensitized gold particles to bind up this antigen ...
SafePath Laboratories

... sorbent treatment (IgM detection) directly in the well. Suitable for automated ELISA systems General information The ELISA method is based upon the reaction of antibodies in the sample tested ...

Result display time: 60 min
Sensitivity: 99 %
... instrument, and the detection results can be obtained within 45 minutes. Novel Coronavirus 2019-nCoV + S Mutant Nucleic Acid Detection Kit Model - Detection of mutation sites - ...
Bioteke Corporation

... quantification of the viral DNA present in the sample The assay shares the same thermal profile of the REALQUALITY Infectious diseases kits Validated on main Real time PCR instruments The ...
AB Analitica

Result display time: 120 min
... The kit allows 96 tests, including controls in a split microtiter plate with color-coded strips and breakable wells. Advantages: The total assay time is about 2 hours. High sensitivity ...
TestLine Clinical Diagnostics

Paves your way to functional analysis of a specific research question. Intended use This FluoroSpot Path kit is intended for the enumeration of cells secreting human IFN-γ, granzyme B, and IL-2 in response to SARS-CoV-2 ...
Mabtech

Result display time: 18 h - 23 h
Sample volume: 0.1 ml
... ELISpot Assay Kit is designed to determine the frequency of CD178 producing cells under a given stimulation and the comparison of such frequency against a specific treatment or pathological state. The ...
Result display time: 75 min
Sample volume: 0.05 ml
... the later which nullifies the gastric acid around the germ. H. pylori screening is essential in the diagnosis of stomach diseases, in that the bacterial infections is associated with stomach disorders characterized by ...
MEDIPAN GmbH
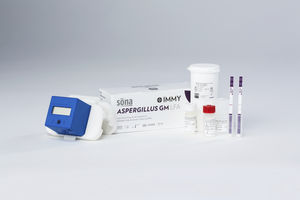
Result display time: 45 min
Specificity: 98, 92 %
Sensitivity: 97, 91 %
Over 30 million people are at risk of invasive aspergillosis each year due to use of corticosteroids, chemotherapy or other immunosuppressive agents, and over 300,000 patients develop it annually. 1 Timely diagnosis and initiation ...
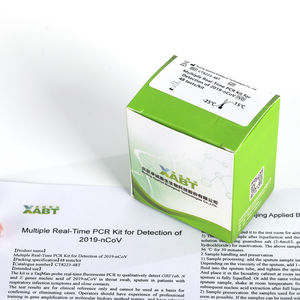
Multiple Real-Time PCR Kit for Detection of 2019-nCoV is a Real-Time RT-PCR test intended for the qualitative detection of nucleic acid from the 2019-nCoV in oropharyngeal swabs, nasopharyngeal swabs ...
BEIJING APPLIED BIOLOGICAL TECHNOLOGIES

Product Name: Epstein-Barr Virus Detection Kit (Fluorescence PCR) Sample Type: Whole blood, serum or plasma Assay Target: EBV nucleic acid Analytical Sensitivity(LoD): 1000 copies/mL Specification: ...
Zhejiang Orient Gene

Sample volume: 0.2 ml
Invitek Molecular strives to produce the highest quality nucleic acid extraction products for infectious diseases, from research to diagnostics. Universal use Simultaneous isolation of viral DNA & ...

【Intended use】 This kit detects HIV-1 virus DNA which is integrated into human genome of white cells from blood samples. The kit can be used for diagnosis and assisted diagnosis of HIV-1 infection at ...

Beijing Tigsun Diagnostics Co,.Ltd.

... varicella zoster virus infections alongside clinical data of the patient and other laboratory tests outcomes. Transport and storage • The reagents and the test can be shipped and ...
VITASSAY HEALTHCARE S.L.
AdenoVirus Test Helicobacter Pylori Test RotaVirus Test Rota/Adeno Virus Test Strep A Test Syphilis Test Tuberculosis ...
Your suggestions for improvement:
the best suppliers
Subscribe to our newsletter
Receive monthly updates on this section.
Please refer to our Privacy Policy for details on how MedicalExpo processes your personal data.
- Brand list
- Manufacturer account
- Buyer account
- Our services
- Newsletter subscription
- About VirtualExpo Group
























Please specify:
Help us improve:
remaining