- Laboratory >
- Laboratory medicine >
- Laboratory test kit
Laboratory test kits


... of antibodies to the Erns protein of the Classical Swine Fever Virus (CSFV) The pigtype CSFV Erns Ab is a double antigen ELISA for the rapid and reliable detection of antibodies to the Erns protein of the Classical ...
INDICAL BIOSCIENCE
ELISA-VIDITEST anti-CMV kits are intended for the diagnosis of diseases associated with CMV infection, e.g. CMV mononucleosis, CMV syndrome, acute and chronic infections in immunocompromised patients. The tests ...
VIDIA s.r.o

Sample volume: 0.005 ml
Specificity: 99.8 %
Sensitivity: 100 %
COVID-19 Coronavirus Real Time PCR Kit is an In Vitro Diagnostic (IVD) reagent applying on fluorescent PCR technology and aiming at qualitatively detect Open Reading Frame gene region (ORF1a/b) and viral ...
Jiangsu Bioperfectus Technologies Co., Ltd.

Result display time: 40 min
... histological analysis and molecular studies. FFPE tissue is widely used in pathology, research, and clinical diagnostics. This kit is intended for manual and automated isolation of genomic DNA from mammalian FFPE tissue ...

BioKits BLG Assay Kit is used for the detection and quantification of β-lactoglobulin (BLG) in food products and environmental swabs by enzyme immunoassay. Specifications Analyte - BLG Platform ...
Result display time: 12 min
... piece Test strip - 1 piece Biosafety Bag - 1 piece Certificate of inspection 1 piece Before testing, please add a timing function device. Wash hands and remove accessories in the test kit. ...
Lansion Biotechnology Co., Ltd

Result display time: 15 min
... in asopharyngeal and nasal swab specimen. The test is used as an aid in the rapid diagnosis of SARS- CoV-2 viral infections. The INCLIX TRF COVID-19 Ag is intended for use by trained laboratory ...
Sugentech, Inc.

Result display time: 1 min
Sample volume: 0.005 ml
Test Kit is for in-vitrodiagnostic (IVD) use to détermine Hemoglobin (Hb) quantitatively in whole blood from a human. It provides valuable information when the abnormal hemoglobin level is detected or ...


sexually transmitted disease test kitCT/NG/UU
Result display time: 30 min
... scénarios: rapid test in yourfacilities of ail kinds • - Detecting multiple pathogens atthe same time: 3 différent STDs are specifically detected in one single reaction; high Accuracy, sensitivity and specificity concordance to ...

Result display time: 15 min - 20 min
Sample volume: 0.02, 0.04 ml
... high requirements for laboratory safety and operator skills. This time-consuming and demanding traditional neutralization detection method does not apply to COVID-19 with fast transmission speed and wide coverage. Therefore, ...


Chlamydia trachomatis detection kitHWTS-UR001A
This kit is used for qualitative detection of Chlamydia trachomatis nucleic acid in male urine, male urethral swab, and female cervical swab samples. Intended Use This kit is used for qualitative ...
Jiangsu Macro micro-test Medical Technology

The kit is used for a step of nucleic acid extraction, enrichment and purification, and the resulting product is used for nucleic acid detection. When 1 aliquot of the nucleic acid extraction buffer is added to 1 aliquot ...

... or Chikungunya virus infections alongside patient clinical data and other laboratory tests outcomes. Transport and storage • The resuspended positive control should be stored at -20ºC. In order to ...
VITASSAY HEALTHCARE S.L.

Result display time: 180 min
Sample volume: 0.1 ml
The ELISA (Enzyme-Linked Immunosorbent Assay) kit is an in vitro enzyme-linked immunosorbent assay for the quantitative measurement of samples in serum, plasma (EDTA, citrate, ). Intended use - This ...
Result display time: 8 min
... Detection time 8 minutes Application department Clinical Laboratory, Cardiology Department, ICU, Emergency Department, etc. Product specification 20 tests/kit ; 25 tests/kit
Nanjing Vazyme Medical Technology Co.,Ltd

Specificity: 100 %
... conjunction with clinical presentation and other laboratory markers. But in order to validate the treatment of hepatitis B and overcomes of the most significant weakness of the standard serological test ...
Advanced Molecular Diagnostics Ltd.

Result display time: 2 h
... B Multiplex Real-Time PCR Kit is intended for use by qualified clinical laboratory personnel specifically instructed and trained in the techniques of real-time PCR assays and in vitro diagnostic procedures. contents Kit ...

Result display time: 100 min - 119 min
Specificity: 99 %
Sensitivity: 100 %
... confidence High volume manufacturing capabilities – Meet global test demands >10,000 tests/hour The kit is intended to use viral RNA samples that have been pre-extracted from specimens ...

Monkeypox Virus Nucleic Acid Detection Kit Features: High sensitivity: Limit of Detection(LoD) <5 × 102 copies/ml With internal control: Monitor the progress of the experiment and avoid false negative Suitable ...

... common symptoms of monkeypox, which can lead to a variety of medical issues. Specimen Collection and Sample Preparation Laboratory personnel with the necessary experience and understanding should collect specimens ...
INOVIALAB

Result display time: 23 min
... animals do not show visible symptoms. And in general, specific laboratory tests are not available for rapid diagnosis of tick-borne diseases. CareDx™ Canine Tick-borne Disease Real-time PCR Kit ...

Result display time: 135 min
The MicroVue Ba EIA measures the amount of Ba present in human urine, serum, plasma and other biological or experimental samples. The alternative complement pathway provides innate protection against microbial agents in the absence ...
Result display time: 8 min - 15 min
Sample volume: 0.0085, 0.005 ml
The Diacegene SARS-CoV-2 Neutralizing Antibody Assay Kit (Immunofluorescence) is used as an aid in identifying individuals with an adaptive immune response to SARS-CoV-2, indicating recent or prior infection, ...
Sichuan Xincheng Biological Co., LTD
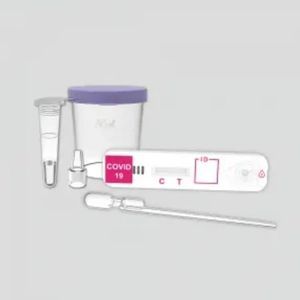
Result display time: 15, 30 min
... appropriate personal protective equipment (PPE). The COVID-19 Antigen Rapid Test has two letters on the surface of the test device indicating test line (T) and control line (C). Test ...

... all relevant clinical and laboratory finding. RTA HBV Real-Time PCR Kit has been validated for use with RTA Viral DNA Isolation Kit or RTA Viral Nucleic Acid Isolation Kit ...
Your suggestions for improvement:
the best suppliers
Subscribe to our newsletter
Receive monthly updates on this section.
Please refer to our Privacy Policy for details on how MedicalExpo processes your personal data.
- Brand list
- Manufacturer account
- Buyer account
- Our services
- Newsletter subscription
- About VirtualExpo Group





















Please specify:
Help us improve:
remaining