- Laboratory >
- Laboratory medicine >
- Parainfluenza test kit
Parainfluenza test kits
{{product.productLabel}} {{product.model}}
{{#if product.featureValues}}{{product.productPrice.formattedPrice}} {{#if product.productPrice.priceType === "PRICE_RANGE" }} - {{product.productPrice.formattedPriceMax}} {{/if}}
{{#each product.specData:i}}
{{name}}: {{value}}
{{#i!=(product.specData.length-1)}}
{{/end}}
{{/each}}
{{{product.idpText}}}
{{product.productLabel}} {{product.model}}
{{#if product.featureValues}}{{product.productPrice.formattedPrice}} {{#if product.productPrice.priceType === "PRICE_RANGE" }} - {{product.productPrice.formattedPriceMax}} {{/if}}
{{#each product.specData:i}}
{{name}}: {{value}}
{{#i!=(product.specData.length-1)}}
{{/end}}
{{/each}}
{{{product.idpText}}}

... Ready-to-use assay mix (all-in-one tube) Product Details To ensure future-proof detection of SARS-CoV-2, we developed a novel assay kit covering six targets across four genomic regions (ORF1ab, ...

... Nucleic Acid Multiplex Detection Kit (Fluorescence PCR Method) Intended Use: A variety of viruses can cause upper respiratory tract infections. The Tianlong Respiratory 12 Types Virus Nucleic Acid ...
Xian Tianlong Science and Technology

... Nucleic Acid Detection Kit (Fluorescence PCR Method) Cat. No.: P232H Specification : 10T/kit(non-prefilled) Specimen: nasopharyngeal swab Storage & Validity: -25℃~-15℃ for 12 months Applicable ...
Xian Tianlong Science and Technology

... Acid Detection Kit (Fluorescence PCR Method) Cat. No.: P232H Specification : 10T/kit(non-prefilled) Specimen: nasopharyngeal swab Storage & Validity: -25℃~-15℃ for 12 months Certificate ...
Xian Tianlong Science and Technology

... eight (8) sample allotments in a 96-test kit, and robust stability are compatible with an existing workflow. Quidel wants to help any molecular laboratory use Lyra to expand their existing test ...
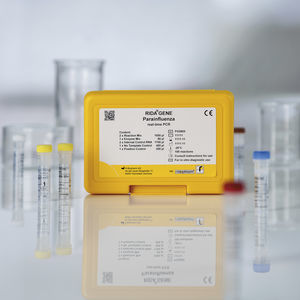
... and symptoms of acute respiratory infection. The RIDA®GENE Parainfluenza test is intended to support the diagnosis of parainfluenza (parainfluenza 1, parainfluenza ...
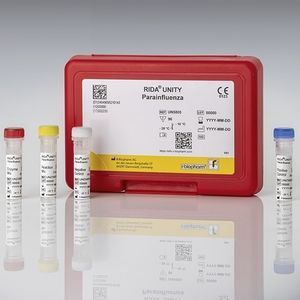
For in vitro diagnostic use. The RIDA®UNITY Parainfluenza test is a multiplex real-time RT-PCR for the direct qualitative detection and differentiation of parainfluenza 1, parainfluenza ...

... single channel on real-time PCR instrument. Allplex™ Respiratory Panel Assays allows faster, more reliable and comprehensive test results than any other products by combination with Seegene’s automation platforms. KEY ...
Seegene

... ; SARS-CoV-2; Variant; Omicron; Flu A, Flu B; RSV; Tuberculosis; Dengue; Respiratory Pathogens Multiple Detection Kit A variety of detection kits provide reliable ...

GenMark’s Respiratory Pathogen Panels identify the most common viral and bacterial organisms associated with upper respiratory infection, including SARS-CoV-2, the virus that causes COVID-19 (on the RP2 Panel). Co-circulation of Pathogens ...
Result display time: 45 min
Specificity: 99.3 %
Sensitivity: 97.1 %
... high efficiency and throughput on the BioFire® FilmArray® 2.0 and the BioFire® FilmArray® Torch Systems. Rapid respiratory PCR test results may enable better-informed diagnosis and treatment of patients. Quick turnaround ...
BioFire Diagnostics

Bosphore SARS-CoV-2 / Flu / RSV Panel Kit is a Real-Time PCR based in-vitro diagnostic medical device, IVD CE marked according to 98/79/EC Directive. Bosphore SARS-CoV-2/Flu/RSV Panel Kit detects ...

The Human Parainfluenza Virus Type l/llI & Adenovirus Detection Kit(Fluorescence PCR) is a real-time RT-PCR test for the simultaneous in vitro qualitative detection and differentiation ...

Respiratory virus multiplex PCR detection kit Features : - To carry out the preliminary screening, identification, and epidemic prevention and control of respiratory infections. ...

... name】Parainfluenza virus type 1 nucleic acid detection kit(PCR-Fluorescent probe method) 【Intended use】 This kit is used for the qualitative detection of parainfluenza ...
hecin-scientific

Result display time: 15 min - 20 min
... important. Respiratory virus six joint test, which includes the détection content is: influenza A virus, influenza B virus, respiratory syncytial virus, Adenovirus, parainfluenza virus type I, and ...
... 2019-nCoV)ORF1ab» N gene • Number of reactions per kit 24 RXNS • PCR platform compatibility Contains FAM, HEXIVIC and Cy5 fluorescence channel Limit Of Detection (Lod): 250copies/ml The Kit ...

This test kit enables the detection of Canine Parainfluenza Virus (CPIV) in the nasal and throat swabs samples of canine using fluorescent RT-PCR. Advantages ✓ Efficient and rapid ...

Result display time: 60 min - 90 min
This kit is used for the combined qualitative detection of SARS-CoV-2, influenza A virus, influenza B virus, adenovirus, mycoplasma pneumoniae, chlamydia pneumoniae, respiratory syncytial virus and parainfluenza ...
Jiangsu Macro micro-test Medical Technology
Result display time: 270 min
Specificity: 100 %
Sensitivity: 97.6 % - 100 %
NeoPlex™ RV-Panel A Detection Kit simultaneously detects 10 viral respiratory pathogens in a single-tube multiplex real-time RT-PCR based on proprietary C-Tag™ technology. Feature of Product Single-Tube ...
Genematrix Inc.

... influenza A and B viruses, respiratory syncytial virus (RSV), parainfluenza viruses (PIV), coronaviruses (CoV), and adenovirus (AdV). The INOGene-Triple Respiratory Panel RT-qPCR Detection kit ...

Human parainfluenza virus IgM antibody test kit (colloidal gold method)
Your suggestions for improvement:
the best suppliers
Subscribe to our newsletter
Receive monthly updates on this section.
Please refer to our Privacy Policy for details on how MedicalExpo processes your personal data.
- Brand list
- Manufacturer account
- Buyer account
- Our services
- Newsletter subscription
- About VirtualExpo Group






















Please specify:
Help us improve:
remaining