- Laboratory >
- Laboratory medicine >
- PCR test kit
PCR test kits
Result display time: 30 min - 40 min
Urinary tract infection is a common high incidence disease worldwide, it has become the second largest infectious disease after respiratory tract infection. It’s high morbidity and mortality is a serious threat to human health and life ...
Bioteke Corporation
Result display time: 240 min
Kit for rapid screening and genotyping of Human Papillomavirus by Single-Step PCR and Reverse Line Blot Allows identification of 40 HPV genotypes:HPV 6, 11, 16, 18, 26, 31, 33, 35, 39, 40, 42, 43, ...
AB Analitica
... Orthopoxvirus genus of the Poxvlridae family. Polymerase chain reaction (PCR) is the preferred laboratory test grven its accuracy and sensitivity. We offer the real-time PCR-based ...

... Name: SARS-CoV-2 Detection Kit (Direct Fluorescence PCR) Sample Type: Oropharyngeal swab or Nasopharyngeal swab Target Gene: ORF1ab Gene, N Gene Analytical Sensitivity(LoD): 200 copies/mL Specification: ...
Zhejiang Orient Gene

The kit is CE certified and intended for in vitro diagnostic use only. Principle The multiplex assay uses fluorescent probes and primers to detect three specific regions within the SARS-CoV-2 nucleocapsid (N) gene ...

Sample volume: 2 ml
... 50 tests/kit 【Expected Usage】 This kit is suitable for the detection of avian influenza virus (AIV) RNA in throat swab,milk and whole blood samples, and the test ...
The SARS-CoV-2 Real-Time RT-PCR Assay is designed for specific and qualitative detection of SARS-CoV-2 RNA in samples such as oropharyngeal swabs, nasopharyngeal swabs or sputum suspected of SARS-CoV-2.

AICON-BIOTM Feline Coronavirus (FCoV) Real-time PCR Kit utilizes real-time PCR technology and premixed freeze drying method to accurately and specifically detect Feline Coronavirus (FCoV) ...

1.A multiplexed test 2.Accuracy and reliability of the results 3.Low sample input Features 1. Simultaneous detection of seven monomorphic homopolymer biomarkersassessment 2. Fast results – 1 hourfrom sample ...

... Specimen: Nasopharyngeal swab Kit Content: Reaction Mix Primer-Probe Mix Internal Control PCR Grade Water Positive Control Compatible Instrument: Bio-Rad (CFX96) LineGene 9600 Series QuantStudio® 5 System Qiagen ...
Medical Innovation Ventures

Hepatitis B virus (HBV) nucleic acid detection kit (fluorescence PCR method) Features: - For in vitro quantitative detection of HBV DNA in human serum or plasma ...
Guangzhou Biotron Technology Co.,Ltd
2019-nCoV/IAV/IBV Nucleic Acid Test Kit (PCR- fluorescence probe method) This kit is a real-time RT-PCR test intended for the qualitative ...
hecin-scientific

This kit is used for qualitative detection of Pneumocystis jirovecii/Aspergillus/cryptococcus.

... of cancer to facilitate targeted therapy. By incorporating Droplet Digital PCR technology, Droplex shows improved sensitivity with wider mutation coverage. Droplex Test Series will provide the possible ...
Gencurix
... amplification of selected DNA fragment with the detection of PCR products in real time. The PCR kits of the “MOLgen” series include a Positive Control sample (PC) corresponding to ...

Intended use: Detection of SARS-CoV-2 specifie RNA Test principle: Real-time reverse transcription polymerase chain reaction and fluorescent probe-primer Sample: Nasopharyngeal swabs(NPS) and sputum Store temperature: ...

... amplification, doubling the efficiency Distinguish high risk type Hpv16,18 independent typing General equipment Used for PCR instruments with 4 fluorescent channels or more Product information Intended use:Nucleic ...

Sample volume: 0.1, 0.4, 1.5, 1 ml
... cervical caner. The test results are only for clinical reference, not for case confirmation or exclusion. Test Principle: The specific primers and specific fluorescent probes are designed for the ...
Biokey Health

... Taylorella equigenitalis (Availability/distribution: Outside the U.S. and Canada) Product details The cador T. equigenitalis PCR Kit is a highly sensitive and specific ready-to-use assay for the ...
... Nucleic Acid Detection Kit (PCR Fluorescence Probe Method) is used for the in vitro qualitative detection of 2019 novel coronavirus (2019-nCoV) RNA in upper respiratory tract specimens ...
Beijing OriginGene-Tech Biotechnology Co.,Ltd

... notoriously; an emerging clinical problem due to treatment limitations for this disease. EasySeq™ 18S and Cyp51A rRNA Fungal ID Kit supports an NGS driven fungal identification strategy for infectious disease, contamination ...
... with TKI resistance, mutations T790M exon 20 result in TKI resistance in about 50% of patients. [intended use] iThis kit is used for qualitative detection of paraffin-embedded pathological tissue or sections, fresh ...
Jiangsu MicroDiag Biomedicine Technology Co., Ltd.

... matter, plants, soil TA cloning “Difficult” PCR – GC/AT rich DNA Colony PCR High throughput PCR Fluorescent dye-based qPCR
GY Highland Biotech
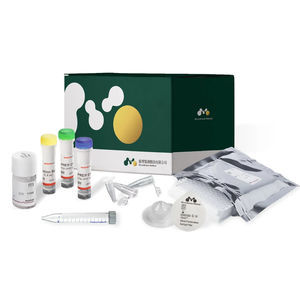

infectious disease test kitDevin™ Microbial DNA Enrichment Kit/24 rxns/RUO [MEK-01-024]
... all platforms, qPCR and end point PCR • With PaRTI-Seq™ enables pathogen identification and reporting within less than 24 Hours Each kit contains: • Devin ™ filter (DSF-01) x 24 pcs • Microbial ...

Result display time: 60 min - 90 min
Specificity: 97 % - 99 %
Sensitivity: 97 % - 99 %
Freeze-dried Dengue Virus I/II/III/IV Nucleic Acid Detection Kit (Fluorescence PCR) [Packing Size] 20 tests/kit, 50 tests/kit [Intended ...
Jiangsu Macro micro-test Medical Technology
Your suggestions for improvement:
the best suppliers
Subscribe to our newsletter
Receive monthly updates on this section.
Please refer to our Privacy Policy for details on how MedicalExpo processes your personal data.
- Brand list
- Manufacturer account
- Buyer account
- Our services
- Newsletter subscription
- About VirtualExpo Group

























Please specify:
Help us improve:
remaining