- Laboratory >
- Laboratory medicine >
- Rapid Clostridium difficile test
Rapid Clostridium difficile tests
{{product.productLabel}} {{product.model}}
{{#if product.featureValues}}{{product.productPrice.formattedPrice}} {{#if product.productPrice.priceType === "PRICE_RANGE" }} - {{product.productPrice.formattedPriceMax}} {{/if}}
{{#each product.specData:i}}
{{name}}: {{value}}
{{#i!=(product.specData.length-1)}}
{{/end}}
{{/each}}
{{{product.idpText}}}
{{product.productLabel}} {{product.model}}
{{#if product.featureValues}}{{product.productPrice.formattedPrice}} {{#if product.productPrice.priceType === "PRICE_RANGE" }} - {{product.productPrice.formattedPriceMax}} {{/if}}
{{#each product.specData:i}}
{{name}}: {{value}}
{{#i!=(product.specData.length-1)}}
{{/end}}
{{/each}}
{{{product.idpText}}}

Result display time: 60 min
... Clostridioides difficile (C. difficile). The Vivalytic Rota-, Norovirus and C. difficile Panel is capable of detecting these three common gastrointestinal pathogens. ...

... bacterial pathogens like Clostridioides difficile (C. difficile) can also be responsible. C. difficile is an anaerobic bacterium found widely in soil and the intestinal ...
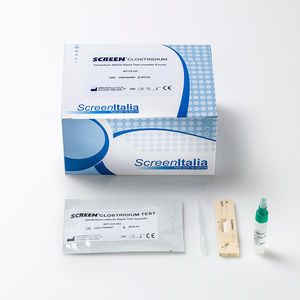
SCREEN TEST CLOSTRIDIUM Clostridium Difficile Gdh Rapid Test REF: SC-0470-25 The Clostridium difficile ...
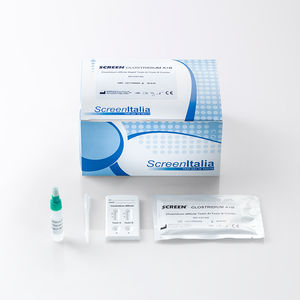
SCREEN TEST CLOSTRIDIUM A + B Clostridium Difficile Toxin A+ Toxin B Rapid Test REF: SC-0494-10 The Clostridium ...


rapid Clostridium difficile testRIDA®QUICK
Result display time: 15 min
Specificity: 95 %
Sensitivity: 100 %
... QUICK Clostridium difficile GDH difficile rapid test improves the reliability of detection of this very consequential nosocomial pathogen when performed ...

Result display time: 45 min
C. difficile infections (CDI) have been increasing in incidence and severity, and are associated with an increase in length of hospital stay, costs, morbidity and mortality.2 Highly virulent (027-NAP1-BI) ...
Cepheid


infectious disease rapid diagnostic testQUIK CHEK®
Result display time: 30 min
Specificity: 92.6 %
Sensitivity: 92.8 %
sobioda

Result display time: 70 min
Sample volume: 0.005 ml
Specificity: 94.2 %
The Lyra Direct C. difficile Assay is a qualitative, multiplexed in vitro diagnostic test for the detection of toxin A gene (tcdA) or toxin B gene (tcdB) sequences of toxigenic strains ...
Quidel Eye Health
Your suggestions for improvement:
the best suppliers
Subscribe to our newsletter
Receive monthly updates on this section.
Please refer to our Privacy Policy for details on how MedicalExpo processes your personal data.
- Brand list
- Manufacturer account
- Buyer account
- Our services
- Newsletter subscription
- About VirtualExpo Group







Please specify:
Help us improve:
remaining