- Laboratory >
- Laboratory medicine >
- Rapid influenza B test
Rapid influenza B tests
{{product.productLabel}} {{product.model}}
{{#if product.featureValues}}{{product.productPrice.formattedPrice}} {{#if product.productPrice.priceType === "PRICE_RANGE" }} - {{product.productPrice.formattedPriceMax}} {{/if}}
{{#each product.specData:i}}
{{name}}: {{value}}
{{#i!=(product.specData.length-1)}}
{{/end}}
{{/each}}
{{{product.idpText}}}
{{product.productLabel}} {{product.model}}
{{#if product.featureValues}}{{product.productPrice.formattedPrice}} {{#if product.productPrice.priceType === "PRICE_RANGE" }} - {{product.productPrice.formattedPriceMax}} {{/if}}
{{#each product.specData:i}}
{{name}}: {{value}}
{{#i!=(product.specData.length-1)}}
{{/end}}
{{/each}}
{{{product.idpText}}}

Suitable for use with the Evidence Investigator from Randox, the Extended Coronavirus Array comprises 6 strains of coronavirus including SARS-CoV-2. The wider panel delivers a more comprehensive respiratory screen allowing differentiation ...
Randox Laboratories

Sample volume: 0.005 ml
Suitable for use with the Evidence Investigator from Randox, the SARS-CoV-2 Array detects both SARS-CoV-2 and Sarbecovirus (confirmatory target), to report COVID-19 positive patient samples.
Randox Laboratories

... results. No laboratory training required. Clinical Significance The Viral Respiratory Tract Infections (VRI) test offers a rapid and comprehensive solution, capable of detecting 10 different viral ...
Randox Laboratories

Result display time: 15 min
Specificity: 90 % - 100 %
Sensitivity: 50 % - 89 %
BinaxNOW™ Influenza A & B Card is an in vitro immunochromatographic assay for the qualitative detection of influenza A and B nucleoprotein antigens in nasopharyngeal ...
Abbott

Qualitative detection of Influenza type A and B antigens from human nasopharyngeal and throat samples

Result display time: 10 min
... QuickVue Influenza A+B Test detects and differentiates influenza type A and type B antigens directly from nasal swab and nasopharyngeal swab specimens. ...

... health Easy testing process Results in minutes Discreetly test at home Iron Test Pack Size: 1 Test Vitamin D Test Pack Size: 1 Test COVID-19 ...

ALLTEST SARS-COV- 2 and Influenza A+B antigen combo rapid test. The COVID-19/Flu A+B Combo Test is a fast and convenient lateral flow ...
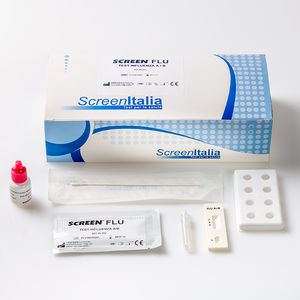
SCREEN TEST FLU A/B Influenza A+B Rapid Test REF: SC-0623-20 Rapid test for the qualitative ...
SCREEN ITALIA
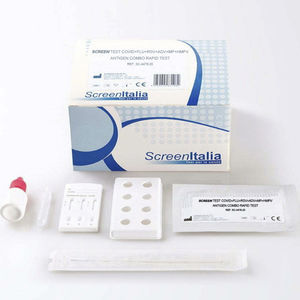
... swabs. This test is intended for professional use only. Screen Test Test Covid+Flu+Rsv+Adv+Mp+Hmpv Combo Test detect SARS-CoV-2, FLU A/B, RSV, ADV, MP ...
SCREEN ITALIA
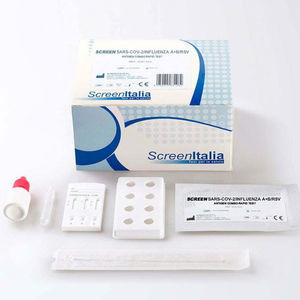
SCREEN TEST COVID-19/INFLUENZA A+B/RSV Antigen Combo Rapid Test REF: SC-2399-20 SARS-CoV-2/Influenza A+B/RSV ...
SCREEN ITALIA

Result display time: 15 min
Sample volume: 0.01 ml
Specificity: 99.8 %
... flow rapid antigen test. It provides quick results within 15 to 20 minutes by detecting specific proteins of SARS-CoV-2, influenza A, influenza B, respiratory ...
Result display time: 10 min
... diagnosis of influenza A and B viral infections. Explain : The Influenza A+B Rapid Test Device is a rapid chromatographic ...
COVID-19 Ag+Flu A/Flu B Rapid Test is used for in vitro qualitative detection of 2019-nCoV antigen, and influenza A/B virus antigens in the human anterior ...

Result display time: 15 min
Specificity: 99.9 %
Sensitivity: 99.9 %
The COVID-19 & Influenza A+B Antigen Combo Rapid Test is a single-use test kit intended for qualitative detection of nucleocapsid protein antigen of ...

Rapid qualitative test that detects influenza type A and type B. 25 Tests per box Description Rapid qualitative test ...

Result display time: 12 min
... near-patient testing Minimal handling steps Test workflow The Instrument and Test Strips are integrated with several quality control checks to ensure the Instrument and Test ...


COVID-19 rapid diagnostic testGenomeCoV19
... SPECIFICITY: No cross-reactivity with influenza A virus, influenza B virus, adenovirus, Staphylococcus epidermidis, HCoV-SARS, HCoV-OC43, HCoV-HKU1, or Streptococcus pneumoniae. See ...

Result display time: 15 min
Sample volume: 0.01, 0.02 ml
Device-free rapid tests provide fast answers regarding the patient's infection status at different point of care locations. Value-added Function •Colloidal gold labelling technology, no device required •Fast, ...

Result display time: 15 min
... -nCoV & Influenza A/B Ag Combo Rapid Test is a colloidal gold immunoassay (CGIA) for the qualitative and differential detection of influenza A, influenza ...


infectious disease rapid diagnostic testR231T050B0C0
... metapneumovirus (hMPV), influenza A virus (IFA), influenza B virus (IFB), enterovirus (EV), parainfluenza virus 1-3 (PIV 1-3), bocavirus (BoV), cytomegalovirus (CMV), EB virus (EB), measles ...
Jiangsu Mole Bioscience

Result display time: 15 min
Specificity: 100 %
Sensitivity: 100 %
Single immunochromatography test for the dual independent detection of Respiratory Influenza A and B Viruses antigens in nasopharyngeal specimens (swab, aspirate and wash). Advantage Sensitive ...

Result display time: 15 min
... culture techniques can take up to 72 hours Rapid visual tests can be subjective, and their reliability depends on the conditions under which they are used. Aids in the rapid differential ...
Result display time: 3 min
Sample volume: 0.035 ml
Specificity: 95.6, 100, 97.5 %
... Percent Agreement - Influenza A | 100.00 % & 99.67 % Percent Agreement - Influenza B | 97.50 % & 98.66 % Percent Agreement Microfluidic Qualitative Immunoassay FREND™ COVID-19 ...
NanoEntek

Result display time: 3 min
... nucleocapsid antigen tests that detects infection caused by SARS-CoV-2 and Influenza A (Flu A) and Influenza B (Flu B). VYRACoV2Flu is the right tool ...

Result display time: 15 min
COVID-19/InfluenzaA+B/RSV/Adenovirus /M.pneumoniae Antigen Combo Rapid Test is a rapid chromatographic immunoassay for the qualitative detection of SARS-CoV-2, Influenza ...
The BIOMED Influenza A+B Rapid Test is an immunological one-step membrane test for the rapid and qualitative determination of influenza ...

... acids assay of influenza A and B viruses from Wuhan Union Hospital (statistics on January 23, 2020) have shown that, the positive rate of influenza A and B nucleic acids ...
BEIJING APPLIED BIOLOGICAL TECHNOLOGIES

Result display time: 15 min
Specificity: 99.7 %
Sensitivity: 98.6 %
... antibodies specific for Influenza A and Influenza B to selectively detect Influenza A and Influenza B antigen in nasal swab, throat ...

... intended to aid in the rapid diagnosis of influenza A, influenza B and/or SARS-CoV-2 infections. This test provides only a preliminary test ...
... qualitative detection of COVID-19, Influenza A virus, Influenza B virus,Respiratory syncytial virus (RSV) , Adenovirus (ADV), M.Pneumoniae(MP), Parainfluenza virus(PIV) and CP antigen ...
Your suggestions for improvement:
the best suppliers
Subscribe to our newsletter
Receive monthly updates on this section.
Please refer to our Privacy Policy for details on how MedicalExpo processes your personal data.
- Brand list
- Manufacturer account
- Buyer account
- Our services
- Newsletter subscription
- About VirtualExpo Group

























Please specify:
Help us improve:
remaining