- Laboratory >
- Laboratory medicine >
- Respiratory disease test kit
Respiratory disease test kits
Result display time: 15 min - 20 min
... (SARS-CoV-2) N protein antigen in human nasal swabs Performance and features 02 Simple Individual test, easy to carry Rapid test kit, easy to perform 03 Convenient Visual ...
Aikang Diagnostics
Result display time: 20 min
Gmate COVID-19 Ag kit is an in vitro diagnostic medical device that qualitatively detects antigens against COVID-19 in the nasopharyngeal smear of patients by immunochromatography. Specification Specimen volume ...
PHILOSYS Co., Ltd.

Result display time: 80 min
Sample volume: 0.012 ml
... fluorescent or above equipment Packing specification: 48 tests/box Detection limitation:100 copies/mL Sample processing method: no extraction Storage: cold chain transportation 220 tests / box size: ...


respiratory infection test kitMS01293
System for the presumptive identification and antimicrobial suceptibility test of the most common bacterial pathogens in Acute Respiratory Infections.
CPM SAS

Detect 19 types of respiratory viruses and 6 types of pneumonia-causing bacteria Respiratory Pathogens Real-time PCR Kit is an in vitro diagnostic test for the qualitative ...

Mytest S. pneumoniae Ag test is a lateral flow chromatographic immunoassay for the qualitative detection of Streptococcus pneumoniae Ag in human urine specimen. It is intended to be used as a screening test ...
Biofootprints Healthcare Pvt Ltd
Result display time: 8 min - 12 min
Specificity: 100, 98.8 %
Sensitivity: 97.4, 90.6 %
STANDARD Q Influenza A&B Test performs qualitative analysis by detecting influenza virus A type and B type antigens in human nasopharynx Advantage Easy handling which does not require specific training Suitable ...
SD BIOSENSOR. INC
... instruments Product matching metal bath Storage conditions and expiration date The lyophilized powder components of the kit should be stored below -20°C, and other components should be stored at room temperature ...
Result display time: 20 min
Specificity: 96.6, 98 %
Sensitivity: 58.3, 80.3 %
Influenza (the flu) is a common viral respiratory infection that causes an illness ranging from mild to severe, and sometimes can be fatal. Influenza testing detects the presence of the virus in a sample of respiratory ...
Beijing O&D Biotech Co., Ltd.

Result display time: 55 min
Sample volume: 0.005 ml
This test comes in ready-to-use mixture and the user can use the reagent without mixing ingredients. Dissolve the dry beads in a diluent followed by adding the pre-extracted DNA, and you can run PCR to ...
Boditech Med Inc.
Short detection: Rapid detection of novel coronavirus. No instrumentation required: No need for instruments and equipment,suitable for rapid screening. Suitable for primary screening: Screening for suspected novel coronavirus infected patients.
Biowin Pharmaceutical
The COVID-19 Antibodies test from Diagnox is an FDA-EUA, C-FDA, CE-IVD, and A-TGA approved test for IgG and IgM antibodies. It is a rapid test device that utilizes lateral flow technology ...

Result display time: 15 min
This kit is used to the qualitative detection of SARS-CoV-2 antigen presents in humanoropharyngeal swab and nasopharyngeal swab. Value-added Function: Ø Colloidal gold labelling technology, no device required; Ø ...

... Detection Kit(probe-based qrt-PCR) The remarkable advantages for our Nucleic Acid Detection kits: 1) Specificity: Sars-Cov-2 can be accurately identified by dual - target (ORF1ab+N), No ...
... in plasma or serum. This kit is used for assisted diagnosis. Model: 96 T / kit Brand: Hunan Runmei Gene Certification: CE, ISO 13485 Product Description SARS-CoV-2 (COVID-19) Neutralizing ...
HUNAN RUNMEI GENE TECHNOLOGY CO.,LTD

19 Respiratory Viruses (RVD 19) Nucleic Acid Detection Kit (Real-Time RT-PCR)
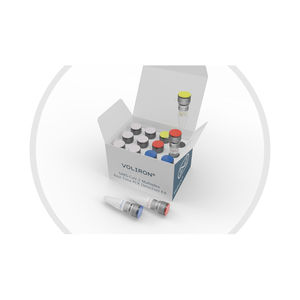
The Voliron® SARS-CoV-2 Real-Time PCR Kit is a multiplex qPCR kit that provides the diagnosis of COVID-19 using oligonucleotides designed to target SARS-CoV-2 specific RdRp and N genes in upper respiratory ...
Synbiotik Biotechnology
Result display time: 10 min - 15 min
Specificity: 100 %
Sensitivity: 98.1, 93.1 %
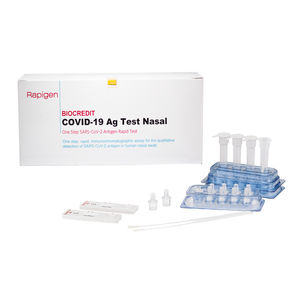
... symptoms. It provides only an initial screening test result and more specific alternative diagnosis methods should be performed in order to confirm COVID-19 infection. Principle of Test BIOCREDIT ...
Biz Distribution
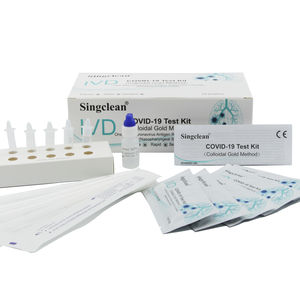
Singclean COVID-19 Test Kit (Colloidal Gold Method) is a solid phase immunochromatographic assay for the rapid, qualitative detection of antigen from COVID-19 in human nasal swab specimen. Features •Results ...
ZHENJIANG LANDFAST
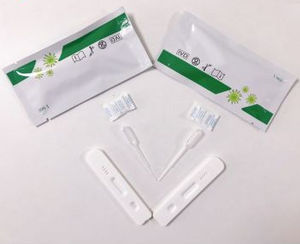
Categories Testing and vaccines Brand gyf Model COVID-19-antigen home self-test kit specification 50pcs/box Certification ce Certification fda Certification cfda accuracy 98% Storage method ...
Tianjin Guyufan Biological Technology
Tuberculosis Antibody(IgG) Detection Kit (ELISA)

Festures: Sterile swabs. Rapid detection :15 minutes out of the results. Sensitivity:98.15% Specificity:98.44% Quick in-home testing takes away the worry of developing symptoms.(Not Available in the U.S)
Sensitivity: 94.5 % - 99 %
As a fact, we cover all cases needed for Covid-19 test via the following alternative options: Swab Sample, Blood Sample or Saliva Sample Anti-gene or Antibody. For Patients with symptoms and for people without ...
Result display time: 10 min - 15 min
Sample volume: 0.01 ml
Specificity: 99, 100 %
COVID-19 IgM/IgG Ab Test is used for qualitative detection of the IgM and IgG antibodies of COVID-19 in human serum/plasma or whole blood.
Result display time: 10 min - 15 min
Specificity: 99.6 %
Sensitivity: 98.1 %
COVID-19 Ag Test is used for qualitative detection of the COVID-19 antigen in human nasopharyngeal or pharyngeal swab specimens.

Result display time: 15 min
The Influenza A+B Test detects and differentiates influenza type A and type B antigens directly from nasal swab and nasopharyngeal swab specimens. A single sample can be used to run both the Influenza A+B Test ...
Your suggestions for improvement:
the best suppliers
Subscribe to our newsletter
Receive monthly updates on this section.
Please refer to our Privacy Policy for details on how MedicalExpo processes your personal data.
- Brand list
- Manufacturer account
- Buyer account
- Our services
- Newsletter subscription
- About VirtualExpo Group























Please specify:
Help us improve:
remaining