- Laboratory >
- Laboratory medicine >
- Sputum test kit
Sputum test kits
{{product.productLabel}} {{product.model}}
{{#if product.featureValues}}{{product.productPrice.formattedPrice}} {{#if product.productPrice.priceType === "PRICE_RANGE" }} - {{product.productPrice.formattedPriceMax}} {{/if}}
{{#each product.specData:i}}
{{name}}: {{value}}
{{#i!=(product.specData.length-1)}}
{{/end}}
{{/each}}
{{{product.idpText}}}
{{product.productLabel}} {{product.model}}
{{#if product.featureValues}}{{product.productPrice.formattedPrice}} {{#if product.productPrice.priceType === "PRICE_RANGE" }} - {{product.productPrice.formattedPriceMax}} {{/if}}
{{#each product.specData:i}}
{{name}}: {{value}}
{{#i!=(product.specData.length-1)}}
{{/end}}
{{/each}}
{{{product.idpText}}}

... Nucleic Acid Multiplex Detection Kit (Fluorescence PCR Method) Intended Use: A variety of viruses can cause upper respiratory tract infections. The Tianlong Respiratory 12 Types Virus Nucleic Acid ...
Xian Tianlong Science and Technology

... pneumoniae. The test results of this kit are for clinical reference only and cannot be used as the basis for diagnosis or exclusion of cases. Product order info: Product Name: Mycoplasma Pneumoniae ...
Xian Tianlong Science and Technology

... pneumoniae. The test results of this kit are for clinical reference only and cannot be used as the basis for diagnosis or exclusion of cases. Product order info: Product Name: Mycoplasma Pneumoniae ...
Xian Tianlong Science and Technology
Respiratory-Borne Pathogen Detection Kit Respiratory infection refers to a disease of infectivity due to the invasion and infection of pathogens into respiratory tract via the nasal cavity, throat, ...

... direct, qualitative detection and differentiation of Legionella pneumophila and Legionella spp. from human tracheal secretion, sputum and bronchoalveolar lavage fluid (BAL). The RIDA®GENE Legionella multiplex real-time ...
R-Biopharm AG
... distinction is drawn between typical and atypical pathogens. Atypical bacteria cannot be cultivated in a regular culture of sputum or blood and also cannot be visualized by gram staining. This makes it difficult to detect ...
R-Biopharm AG

... multiplex real-time PCR for the direct, qualitative detection of Adenovirus in human stool samples, throat rinsing fluid, sputum and bronchoalveolar lavage (BAL). The RIDA®GENE Adenovirus real-time PCR is intended ...
R-Biopharm AG

Result display time: 2 min
Specificity: 98 %
Sensitivity: 100 %
... Complete respiratory infections menu - Complete respiratory infections range with Aspergillus spp ELITe MGB® Kit, Pneumocystis ELITe MGB® Kit, Respiratory Viral ELITe MGB® Panel and Respiratory Bacterial ...
Several epidemiologists and CDC officials recommended the Respiratory Pathogens Multiplex PCR test for flu season. Positive results from this kits are indicative of the presence of SARS-CoV-2, Influenza ...
Yaneng Bioscience (Shenzhen) Co., Ltd.

... single channel on real-time PCR instrument. Allplex™ Respiratory Panel Assays allows faster, more reliable and comprehensive test results than any other products by combination with Seegene’s automation platforms. KEY ...
Seegene
Result display time: 15 min
This device is intended to be used for the in vitro qualitative detection of 5 items in human alveolar lavage fluid, sputum, nasal swab, nasopharyngeal swab or oropharyngeal swab related to the respiratory infection, ...

This covid-19 PCR test kit is used for in vitro qualitative detection of the ORF1ab and N genes of novel coronavirus (2019-nCoV) from throat swabs and sputum samples in pneumonia suspected ...
DaAn Gene
2019-Novel Coronavirus, Influenza A and B Virus Nucleic Acid Test Kit This kit is intended for the qualitative detection of ORF1ab gene of 2019-Novel Coronavirus (2019-nCoV, SARS-COV-2), ...
Jiangsu Mole Bioscience
Specificity: 100 %
EliGene® COVID19 BASIC A RT kit is intended for the detection of SARSCoV-2 viral RNA from nasopharyngeal swabs, buccal swabs, sputum, saliva, serum, plasma and other clinical samples by one-step RT-qPCR ...
Elisabeth Pharmacon spol


tuberculosis test kitGeneFinder™
Result display time: 90 min
... carryover contamination Reliable result by internal/positive/negative control Easy to use master mix type Including complete assay kits CE-IVD / Ministry Food and Drug Safety (Korea) approved

... viral RNA, which will be detectable in patients before antibodies form or symptoms of the disease are present, which means the test results can tell whether or not that someone gets virus very early on in their illness. PERFORMANCE One-tube/fast ...
Sansure Biotech

Result display time: 90 min
... RT-PCR assay En-swer™ COVID-19 RT-PCR Kit The En-swer™ COVID-19 RT-PCR Kit is an in-vitro diagnostic medical device using 1-step RT-qPCR(real-time reverse-transcription polymerase chain reaction) ...
Protonion™ 96 Viral RNA Kit (K-4731) is designed to accurately and quickly extract viral RNA from clinical samples such as Sputum, Saliva Nasopharyngeal swab, and Oropharyngeal swab using ExiPrep™ 96 ...

Bosphore Novel Coronavirus (2019-nCoV) Detection Kit is a Real-Time PCR based in-vitro diagnostic medical device, IVD CE marked according to 98/79/EC Directive. Bosphore Novel Coronavirus (2019-nCoV) ...
Result display time: 1 h
Specificity: 99.1 %
Sensitivity: 100 %
... transcriptase (RT) polymerase chain reaction (PCR) test intended for the qualitative detection of ORF1ab and N genes of the SARS CoV 2 in nasopharyngeal ( NP) swab, oropharyngeal (OP) swab and sputum ...
Result display time: 52 min
... real-time RT-PCR reagent kit to allow laboratories to diagnose the disease caused by 2019-nCov infection with fast and easy to use assays. Shenzhen Uni-medica RT PCR detection kit is ...

... mid-turbinate swabs, nasal washes, nasal aspirates, sputum and bronchoalveolar lavage fluid (BALF) specimens from individuals suspected of COVID-19 by their healthcare provider. Size 48 Tests/Kit, ...

Result display time: 60 min
Sensitivity: 99 %
... false negative detection results. B.Intended Use This kit is used to achieve qualitative detection of SARS-CoV-2 viral RNA extracted from nasopharyngeal swabs, oropharyngeal swabs and sputum of suspected ...
Bioteke Corporation
Multiple Real-Time PCR Kit for Detection of 2019-nCoV is a Real-Time RT-PCR test intended for the qualitative detection of nucleic acid from the 2019-nCoV in oropharyngeal swabs, nasopharyngeal swabs ...

Product Name: SARS-CoV-2 Detection Kit (Direct Fluorescence PCR) Sample Type: Oropharyngeal swab or Nasopharyngeal swab Target Gene: ORF1ab Gene, N Gene Analytical Sensitivity(LoD): 200 copies/mL Specification: ...

The kit is CE certified and intended for in vitro diagnostic use only. Principle The multiplex assay uses fluorescent probes and primers to detect three specific regions within the SARS-CoV-2 nucleocapsid (N) gene ...
Sample volume: 0.005 ml
The SC2 Real-Time PCR Detection Kit is designed for specific and qualitative detection of SARS-CoV-2 RNA in samples such as oropharyngeal swabs, nasopharyngeal swabs or sputum suspected ...
Biomiga, Inc.
Specificity: 100 %
Sensitivity: 100 %
The COVID-19 Real-Time PCR Test is designed for specific and qualitative detection of the novel coronavirus SARS-CoV-2, responsible for COVID-19, in oropharyngeal swabs, nasopharyngeal swabs or sputum ...
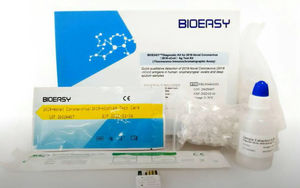
Result display time: 10 min
FLUORESCENCE IMMUNOCHROMATOGRAPHIC ASSAY AG TEST KIT Focused and dedicated to the rapid test, PCR and FIA technology development for the medical industry, Bioeasy offer the PCR, FIA ...
Your suggestions for improvement:
the best suppliers
Subscribe to our newsletter
Receive monthly updates on this section.
Please refer to our Privacy Policy for details on how MedicalExpo processes your personal data.
- Brand list
- Manufacturer account
- Buyer account
- Our services
- Newsletter subscription
- About VirtualExpo Group


























Please specify:
Help us improve:
remaining