- Secondary care >
- Neurology >
- Transcutaneous neurostimulator
Transcutaneous neurostimulators
{{product.productLabel}} {{product.model}}
{{#if product.featureValues}}{{product.productPrice.formattedPrice}} {{#if product.productPrice.priceType === "PRICE_RANGE" }} - {{product.productPrice.formattedPriceMax}} {{/if}}
{{#each product.specData:i}}
{{name}}: {{value}}
{{#i!=(product.specData.length-1)}}
{{/end}}
{{/each}}
{{{product.idpText}}}
{{product.productLabel}} {{product.model}}
{{#if product.featureValues}}{{product.productPrice.formattedPrice}} {{#if product.productPrice.priceType === "PRICE_RANGE" }} - {{product.productPrice.formattedPriceMax}} {{/if}}
{{#each product.specData:i}}
{{name}}: {{value}}
{{#i!=(product.specData.length-1)}}
{{/end}}
{{/each}}
{{{product.idpText}}}

EMS Neurostimulator is a stand alone, innovative, multi channel unit for cortical stimulation. It is used for electrical cortical stimulation, direct nerve stimulation and muscle stimulation. The EMS Neurostimulator ...

Galvanic Vestibular Stimulation (Bench-top form factor)

The world is changing so are treatments! tVNS® E is a medical device, CE Certified according to MDR EU 2017/745, indicated for treatment of epilepsy, depression, autism, anxiety, atrial fibrillation, Crohn’s disease(IBD), cognitive impairment, ...

Transcutaneous Vagus Nerve Stimulation (tVNS®) Device for Research Main Features : Full Wireless Parameter Programmability Patient App for Data Collection Support for Clinical Trials Triggered tVNS® Legacy and Hook Electrode Sham ...

Minimally-invasive administration Significant reduction in treatment complexity Potential reduction in long-term treatment costs Easy application Protected from splashes Prescribed by a physician No active substances Enhancement ...
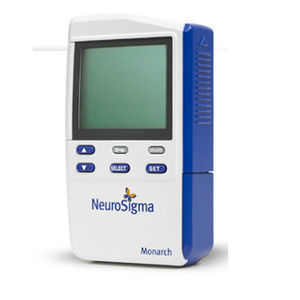
The Monarch eTNS System is indicated for the treatment of pediatric Attention Deficit Hyperactivity Disorder (ADHD) as a monotherapy in patients ages 7 through 12 years old who are not currently taking prescription ADHD medications. The ...

The “Ducest Neurostimulator V” (DNS-V) is a minimally invasive Class 2a neurostimulator that is approved for the treatment of peripheral arterial disease ( PAD ) and pain. The DNS-V device can be ...

Treat primary headache without taking any medicines through gammaCore® therapy. GammaCore® therapy is a non-invasive Neurostimulation Technology that stimulates the nerves using proprietary electrical signals to treat primary headaches, ...


transcutaneous neurostimulatorRelivion®
Relivion MG goes beyond relieving migraine pain and associated symptoms with a complete program to support the management of migraine disease. THE RELIVION DEVICE Relivion is a non-drug, wearable neuromodulation therapy that ...

... advanced, non-invasive, nerve stimulation device that is covered by 18 U.S. utility patents. It is the only wearable neurostimulator that is enabled by a custom designed microchip that provides flexible, precise, high-power ...

Fibromyalgia is a complex health condition that is difficult for patients and challenging for physicians. Quell® Fibromyalgia helps reduce symptoms in patients with fibromyalgia and high pain sensitivity. Wearable neuromodulation technology Quell ...

Low frequency electrical stimulator, a part of XFT-2001D Foot Drop System. Features 10 training scenarios Core technology Working Principle XFT-2001P Peripheral Nerve Stimulator is a low frequency electrical stimulator. It ...

The first FDA-cleared medical device for the reduction of opioid withdrawal symptoms,* Bridge is designed to give your patients the hope they need to continually progress through treatment—without the fear of withdrawal. Proven to ...
Your suggestions for improvement:
the best suppliers
Subscribe to our newsletter
Receive monthly updates on this section.
Please refer to our Privacy Policy for details on how MedicalExpo processes your personal data.
- Brand list
- Manufacturer account
- Buyer account
- Our services
- Newsletter subscription
- About VirtualExpo Group













Please specify:
Help us improve:
remaining