- Medical Technical Facilities >
- Healthcare IT, Telemedicine >
- Validation software
Validation software

SIRxpert™ is an IT platform used to manage all data and results from a clinical microbiology laboratory. Productivity and simplicity – The SIRxpert™ middleware enables you to centralise your manual results and those from your automated ...
i2a (intelligence artificielle applications) S.A.
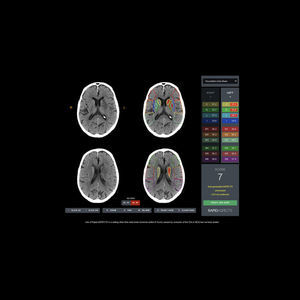
Automatic Identification of ASPECTS Regions and Scores The first neuroimaging device which has been shown to improve reader diagnosis under the FDA’s CADx classification, Rapid ASPECTS is based on the Alberta Stroke Program Early CT ...
RapidAI

Designed by microbiologists for microbiologists, to address the specific needs of microbiology laboratories A software solution fully dedicated to the discipline, which offers customizable analytical protocols and ...

The ValSuite Software ValSuite Pro is an intuitive validation software which collects and presents validation data from all Ellab measuring devices. The software ...

... focused graphical user interface for FOBA laser marking equipment. The PlugIn is directly integrated in FOBA's MarkUS marking software, and has been designed for improving production efficiency, reducing costs and increasing ...
FOBA Laser Marking + Engraving, ALLTEC GmbH
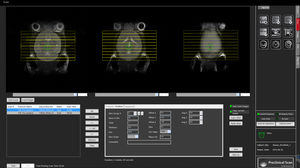
... able to vary many imaging parameters within each MRI pulse sequence. To permit this, a key feature of this interface is the validation for viability/safety of all combinations of sequence parameters selected. The package ...

... RETINOPATHY DETECTION AND GRADING PLATFORM (DR) EyeArt™ is a fully automated, CE marked DR screening software. EyeArt™ is an Artificial Intelligence software platform featuring cutting–edge image ...

Our specialists in medical communication standards have both theoretical knowledge and years of hands-on experience when it comes to interfaces in the clinical field. We would love to inject our expertise into your projects. We can tailor ...

CE marked, FDA cleared, NMPA approved • High Generalization Ability and Rigorous Clinical Validation. • Efficient Analysis and High Sensitivity: effective for small nodules, improving efficiency and accuracy. • Intelligent ...
Beijing Infervision Technology Co. Ltd.

... selected pharmaceutical standard. The software also permits to define house intern tests procedures. An automatic data backup can be stored in a preselected location. A software validation ...

PharmaTrac® is a BPOC – barcode-point-of-care solution designed to ensure patient safety through the validation of medication administration. Bedside Validation Significantly reduces administration ...
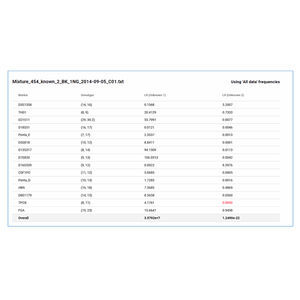
... Probabilistic Mixture Analysis Software. MaSTR software features a rapid and transparent approach to Probabilistic Mixture Analysis which utilizes your forensic acumen in an easy-to-use Windows® environment ...

... machines. Join the RaySafe Service Program by filling out the RaySafe Service Request Form and enjoy the following benefits: Validation/calibration on a fixed schedule Extended service program warranty, ensuring your ...

... administrative features for compliance with FDA regulations. An additional Software Validation Package is available to validate data calculations produced by the Octet® software. Design ...

... required process validation with the help of the powerful reporting and evaluation software Winlog.validation. The automatic recognition of the individual process steps makes your ...

... all the necessary purification, software capabilities, qualification documentation and online support services necessary for the validation required to meet Good Manufacturing Practice (GMP). It includes ...
XpertLog® Software controls all systems manufactured by Lives International®: real time wireless data loggers, standard wireless data loggers, standard data loggers, continuous monitoring data loggers, mapping wireless ...

... bank management system for hospitals and blood establishments. It uses BLOODSPACE RFID together with a compatible blood bank software to track and trace blood components within a hospital’s Critical Control Points (CCP), ...

... testing, and distribution. 21 CFR Part 11 compliant software refers to software systems that meet the criteria set forth in the regulation. These criteria include requirements for: Security: the ...
Your suggestions for improvement:
the best suppliers
Subscribe to our newsletter
Receive monthly updates on this section.
Please refer to our Privacy Policy for details on how MedicalExpo processes your personal data.
- Brand list
- Manufacturer account
- Buyer account
- Our services
- Newsletter subscription
- About VirtualExpo Group





















Please specify:
Help us improve:
remaining