- Primary care
- Emergency medicine, Resuscitation
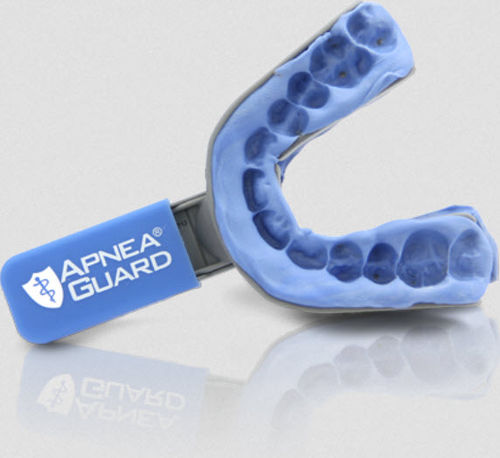
- Mouthpiece anti-snoring mouthpiece
- Advanced Brain Monitoring
Mouthpiece anti-snoring mouthpiece Apnea Guard®

Add to favorites
Compare this product
fo_shop_gate_exact_title
Characteristics
- Type
- mouthpiece
Description
Apnea Guard is a patented non-custom oral appliance that can be fitted in less than 15 minutes by any trained health professional and worn for up to 30 nights. The patented design utilizes a combination of tongue size and sex to select phenotypical features that achieve treatment outcomes superior to the conventional fitting of custom oral appliances.
Apnea Guard was developed as a result of a $1.05 mm NIH grant investigation into how to improve oral appliance therapy outcomes. Research conducted with the Apnea Guard recently received a Clinical Excellence Award and two Clinical Research awards from the American Academy of Dental Sleep Medicine.
The Apnea Guard Theragnostic Protocol explains how the feature characteristics that contributed to its superior efficacy can be digitally scanned and incorporated into custom oral appliance.
Features
1mm incremental advancements across full range of protrusive settings
Three vertical sizes (low, med, high VDOs) proven to improve outcomes in men and women
Systematic approach which predicts the target end-point for 80% of OAT responders
Clinical Applications
Inexpensively identify responders to oral appliance therapy
Reduce OSA symptoms identified post-operatively
Key Finding
79% of patients intolerant of CPAP exhibited a treatment response to the Apnea Guard
If a patient has an AHI < 10 with the Apnea Guard, the odds of custom oral appliance achieving an AHI < 10 is 4.3
Using the Theragnostic Protocol, the Apnea Guard Trial appliance delivered superior treatment outcomes as compared to a conventional fitted custom oral appliance
VIDEO
Catalogs
Apnea Guard
14 Pages
Related Searches
*Prices are pre-tax. They exclude delivery charges and customs duties and do not include additional charges for installation or activation options. Prices are indicative only and may vary by country, with changes to the cost of raw materials and exchange rates.



