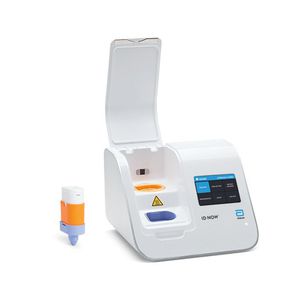
PCR POC analyzer m-PIMA™AIDSfor antigensHIV

Add to favorites
Compare this product
Characteristics
- Analysis mode
- for PCR
- Applications
- AIDS
- Tested parameter
- for antigens
- Micro-organism
- HIV
- Sample type
- blood
- Configuration
- portable
Description
Fully automated nucleic acid testing platform combines the accuracy of molecular testing with a short turnaround time, allowing same day treatment decisions and consultations with patients. The portable m-PIMA™ Analyser brings HIV early infant diagnosis and HIV viral load testing to a wide range of decentralized settings, bypassing delays and inefficiencies associated with centralized testing.
The m-PIMA™ HIV-1/2 VL test is now commercially available and has received CE-IVD marking and WHO prequalification. This product is not available in the United States.
BENEFITS
Actionable results at the point of care, while the patient is still present
Empower decentralized decision making
Engage patients on their diagnosis and treatment
Patients lost to follow up potentially reduced
Complement central laboratory infrastructure
Manage decentralized programmes via built-in connectivity
VIDEO
Catalogs
No catalogs are available for this product.
See all of Abbott‘s catalogsRelated Searches
- Assay kit
- Blood assay kit
- Immunoassay assay kit
- Blood rapid diagnostic test
- Rapid lateral flow test
- Immunoassay rapid diagnostic test
- Cassette rapid diagnostic test
- Virus rapid diagnostic test
- Whole blood detection kit
- Serum rapid diagnostic test
- Plasma rapid diagnostic test
- Infectious disease rapid diagnostic test
- Whole blood rapid diagnostic test
- Cassette assay kit
- Rapid respiratory infection test
- Urine rapid diagnostic test
- COVID-19 rapid diagnostic test
- Bacteria rapid diagnostic test
- Oncology test kit
- Strip rapid diagnostic test
*Prices are pre-tax. They exclude delivery charges and customs duties and do not include additional charges for installation or activation options. Prices are indicative only and may vary by country, with changes to the cost of raw materials and exchange rates.