- Laboratory
- Laboratory medicine
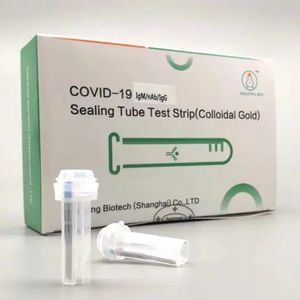
- COVID-19 test strip
- Amazing Biotech Co., Ltd

- Products
- Catalogs
- News & Trends
- Exhibitions
COVID-19 test strip for antigensSARS-COV-2clinical
Add to favorites
Compare this product
Characteristics
- Applications
- COVID-19
- Tested parameter
- for antigens
- Micro-organism
- SARS-COV-2
- Sample type
- clinical, nasal
- Analysis mode
- immunochromatographic
Description
The kit is intended for symptomatic individuals within first seven days from onset of the symptoms. It is for the qualitative detection of SARS-associated corona virus (SARS-CoV-2) antigens and Influenza A&B antigens from human nasal swab to assist in the diagnosis of corona virus (Covid-19) infection.
It is recommended to combine results from this test with further clinical data to confirm the diagnosis.
TYPES OF SPECIMENS
Human nasal swab.
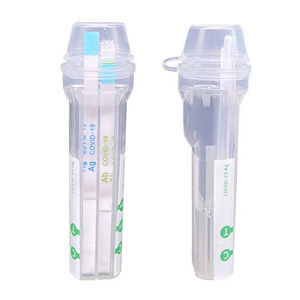
PRINCIPLE
It is based on a sandwich method immunochromatographic assay for the highly sensitive detection of COVID-19 nucleoprotein and Influenza A&B in human nasal providing with an invisible test area (T) and control area (C). Then a specimen is released into the tube buffer, the liquid laterally migrates along the test strip. If sufficient COVID-19 and Influenza A&B antigen is present in the specimen, a visible line of test area (T) appears. As process control, a colored band shall appear in the control area (C) to confirm that sufficient specimen has been absorbed. The test result is visually interpreted after 15 minutes basing on the presence or absence of visually recognizable colored lines. The strip signals could last 10 days for double check if necessary.
REAGENTS AND MATERIALS PROVIDED
Each kit contains buffer, swab and an instruction manual.
VIDEO
Catalogs
No catalogs are available for this product.
See all of Amazing Biotech Co., Ltd‘s catalogsRelated Searches
- Assay kit
- Blood assay kit
- Serum assay kit
- Immunoassay assay kit
- Plasma assay kit
- Whole blood detection kit
- Respiratory infection test kit
- Optical assay kit
- Clinical assay kit
- Lateral flow test kit
- COVID-19 detection kit
- Antigen assay kit
- IgG test kit
- Strip detection kit
- Test strip
- Laboratory detection kit
- Nasopharyngeal assay kit
- Oral fluid test kit
- Coronavirus detection kit
- Nasal detection kit
*Prices are pre-tax. They exclude delivery charges and customs duties and do not include additional charges for installation or activation options. Prices are indicative only and may vary by country, with changes to the cost of raw materials and exchange rates.