- Laboratory
- Sample management
- Hollow-fiber filter
- Asahi Kasei Medical

- Products
- Catalogs
- News & Trends
- Exhibitions
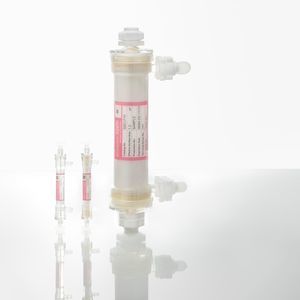
Antiviral filter Planova™ 15Nhollow-fiberplasmalaboratory
Add to favorites
Compare this product
Characteristics
- Filter type
- antiviral, hollow-fiber
- Fluid
- plasma
- Applications
- laboratory
- Material
- polycarbonate
Description
As the world's first filters developed specifically for removing viruses from biotherapeutic drug products, Planova filters have been leading the virus filtration industry. The consistent performance and quality of Planova filters in nearly 30 years of time have contributed to the reputation of these filters as the reliable and dependable virus filters in ensuring viral safety of biopharmaceutical drug products.
Applications
First launched in 1989, Planova filters containing regenerated cellulose hollow fiber membranes have a long history of application in biopharmaceutical industry. The filters have been trusted by customers worldwide to ensure viral safety of various biopharmaceutical products: from plasma-derived molecules such as immunoglobulins and coagulation factors, to recombinant proteins such as monoclonal antibodies. It is the pioneer and highly reliable virus filter for biopharmaceutical development.
Features & Performances
Accumulated Trust for Wide Range of Application
Planova 15N, 20N and 35N filters have been trusted by customers for a long time to ensure viral safety of their biopharmaceutical products, simply because they work for various protein molecules.
The unique hollow fiber membrane structure empowers the excellent filterability of Planova filters. Based on size exclusion mechanism, membrane structure of the filters allows proteins to easily pass through the hollow fiber walls without adsorption or denaturation, while viruses are efficiently captured in the membrane pores.
Catalogs
No catalogs are available for this product.
See all of Asahi Kasei Medical‘s catalogs*Prices are pre-tax. They exclude delivery charges and customs duties and do not include additional charges for installation or activation options. Prices are indicative only and may vary by country, with changes to the cost of raw materials and exchange rates.