- Laboratory
- Sample management
- Vial capping system
- AWS BIO PHARMA TECHNOLOGIES

- Products
- Catalogs
- News & Trends
- Exhibitions
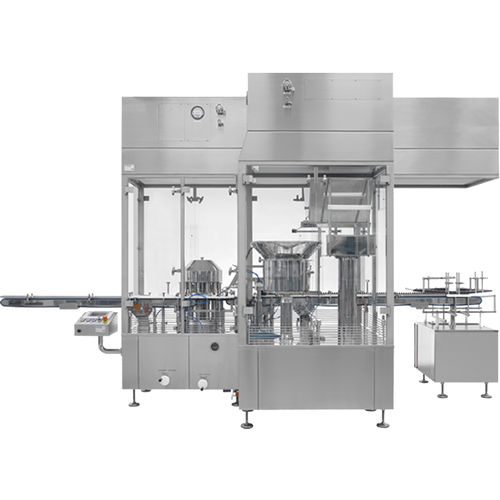
Vial capping system for the pharmaceutical industryrotaryin-line

Add to favorites
Compare this product
Characteristics
- Product applications
- for vials
- Use domain
- for the pharmaceutical industry
- Operation
- rotary, in-line, pressure
- Other characteristics
- floor-standing
Description
OUR ASEPTIC CAPPING SYSTEMS
Our Aseptic Capping Systems process vials, cartridges, syringes and ampoules
Moreover, in-line transport and small width reduces the volume to be monitored and sterilized
Reduced particle generation
Continuous monitoring of the viable and non-viable particles
Vibration Generator for the bowl of aluminum caps
Seal flip-off or tear-off caps (optional)
Install different closing stations (optional)
Further, optional monitoring of the applied pressure during the sealing process is available
In-line control for cap presence detection
Optional standard protection hood
Lastly, all Systems are in compliance with cGMP, GAMP and 21CFR Part11 requirements
VIDEO
Other AWS BIO PHARMA TECHNOLOGIES products
Aseptic Fill-Finish Systems
*Prices are pre-tax. They exclude delivery charges and customs duties and do not include additional charges for installation or activation options. Prices are indicative only and may vary by country, with changes to the cost of raw materials and exchange rates.








