- Secondary care
- Cardiology
- Vena cava filter
- Bard Medical
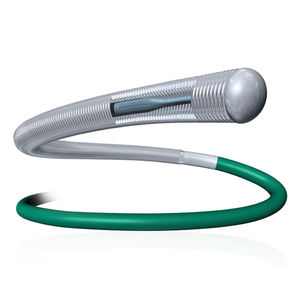
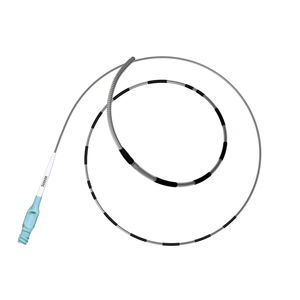
Vena cava filter SIMON NITINOL®
Add to favorites
Compare this product
Description
Features:
Indicated for permanent placement
First approved nitinol vascular implant
Over two decades of performance
2120F - SIMON NITINOL® Vena Cava Filter, Femoral Delivery Kit - 1
2220J - SIMON NITINOL® Vena Cava Filter, Jugular Delivery Kit - 1
2320A - SIMON NITINOL® Vena Cava Filter, Antecubital Delivery Kit - 1
3120F - SIMON NITINOL® Vena Cava Filter, Femoral Introducer Set - 1
3220J - SIMON NITINOL® Vena Cava Filter, Jugular Introducer Set
Simon Nitinol® Vena Cava Filter
Indications for Use: The Simon Nitinol® Filter is indicated for use in
the prevention of recurrent pulmonary embolism via placement in the
vena cava in the following situations: • Pulmonary thromboembolism
when anticoagulants are contraindicated • Failure of anticoagulant
therapy in thromboembolic disease • Emergency treatment following
massive pulmonary embolism where anticipated benefits of
conventional therapy are reduced • Chronic, recurrent pulmonary
embolism where anticoagulant therapy has failed or is contraindicated.
Contraindications for Use: The Simon Nitinol® Filter should not be
implanted in: • Pregnant patients when fluoroscopy may endanger the
fetus • Patients with vena cava diameters greater than 28 mm
• Patients with vena cava diameters greater than 24 mm if those
patients are scheduled for surgery requiring general anathesia within
two weeks • Patients with risk of septic embolism
Catalogs
No catalogs are available for this product.
See all of Bard Medical‘s catalogsRelated Searches
- Catheter
- Balloon catheter
- Stent
- Dilatation catheter
- Cardiac catheter
- Peripheral catheter
- Metal stent
- Catheter guidewire
- Diagnostic catheter
- Venous catheter
- Access catheter
- Central venous catheter
- Hydrophilic guidewire
- Blood vessel catheter
- PTA catheter
- Nitinol stent
- Triple-lumen catheter
- Self-expanding stent
- Vascular introducer
- Balloon catheter pump
*Prices are pre-tax. They exclude delivery charges and customs duties and do not include additional charges for installation or activation options. Prices are indicative only and may vary by country, with changes to the cost of raw materials and exchange rates.