- Secondary care
- Cardiology
- PTA catheter
- Bard Medical
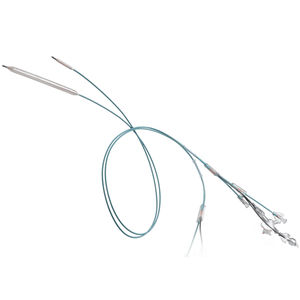
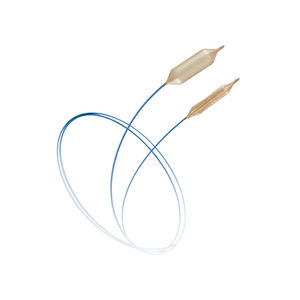
PTA catheter ATLAS® PTAblood vesselballoon
Add to favorites
Compare this product
Characteristics
- Application
- PTA
- Area of the body
- blood vessel
- Options
- balloon
Description
Features
Delivers maximum forces to areas of most resistance
Protects with ultra non-compliant technology
Virtually no balloon growth
Predictable balloon diameters
Reduces risk of overdilatation
Delivers High and Low pressure angioplasty
Largest working range enables lesion specific treatment
Indications for Use: Atlas® PTA Balloon Dilatation Catheters are recommended
for use in Percutaneous Transluminal Angioplasty of the iliac arteries and for the
treatment of obstructive lesions of native or synthetic arteriovenous dialysis fistulae.
This catheter is not for use in coronary arteries.
Contraindications: None known.
Warnings: 1) Contents supplied STERILE using ethylene oxide (EO). Non-Pyrogenic.
Do not use if sterile barrier is opened or damaged. Single patient use only. Do
not reuse, reprocess or re-sterilize. 2) This device has been designed for single
use only. Reusing this medical device bears the risk of cross-patient contamination
as medical devices - particularly those with long and small lumina, joints, and/
or crevices between components - are difficult or impossible to clean once body
fluids or tissues with potential pyrogenic or microbial contamination have had
contact with the medical device for an indeterminable period of time. The residue
of biological material can promote the contamination of the device with pyrogens
or microorganisms which may lead to infectious complications.
Catalogs
No catalogs are available for this product.
See all of Bard Medical‘s catalogsRelated Searches
- Catheter
- Balloon catheter
- Stent
- Dilatation catheter
- Cardiac catheter
- Peripheral catheter
- Metal stent
- Catheter guidewire
- Diagnostic catheter
- Access catheter
- Venous catheter
- Central venous catheter
- Hydrophilic guidewire
- Blood vessel catheter
- PTA catheter
- Nitinol stent
- Triple-lumen catheter
- Self-expanding stent
- Vascular introducer
- Balloon catheter pump
*Prices are pre-tax. They exclude delivery charges and customs duties and do not include additional charges for installation or activation options. Prices are indicative only and may vary by country, with changes to the cost of raw materials and exchange rates.