- Secondary care
- Cardiology
- PTA catheter
- Bard Medical
Access catheter LUTONIX® 018PTAblood vesselballoon
Add to favorites
Compare this product
Characteristics
- Application
- access, PTA
- Area of the body
- blood vessel
- Options
- balloon
Description
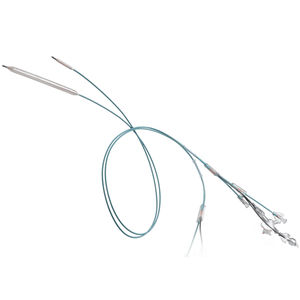
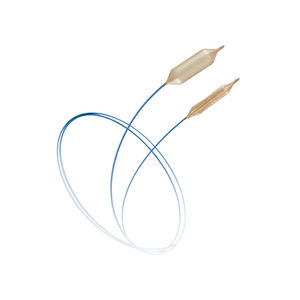
LUTONIX® 018 Drug Coated Balloon PTA Catheter
Deliver More with the Only 018 DCB
Features
The LUTONIX® 018 DCB is designed to:
Perform over small guidewires (up to .018”)
Reduce guidewire exchanges
Enable alternative access
Built on the proven ULTRAVERSE™ 018 platform, LUTONIX® 018 features:
Enhanced Pushability – Reinforced inner lumen provides axial strength
Improved Visibility – Larger, dual distal marker bands on long lengths
GeoAlign® Marking System – Facilitates simple repeat catheter alignment at the lesion
With a crossing profile 20% lower than the lowest profile 035 DCB1, LUTONIX® 018 was built to:
Cross tight lesions
Navigate tortuous anatomy
Reliably deliver drug to complex lesions
Indications for Use: The LUTONIX® 018 Drug Coated Balloon PTA catheter is indicated for
percutaneous transluminal angioplasty, after appropriate vessel preparation, of de novo, restenotic,
or in-stent restenotic lesions up to 300mm in length in native superficial femoral or popliteal arteries
with reference vessel diameters of 4-7mm.
The LUTONIX® 018 Drug Coated Balloon PTA catheter is indicated for percutaneous transluminal
angioplasty, after pre-dilatation, for treatment of stenotic lesions of dysfunctional native
arteriovenous dialysis fistulae that are 4 mm to 7 mm in diameter and up to 80 mm in length.
Contraindications: The LUTONIX® Catheter is contraindicated for use in: 1) Patients who cannot
receive recommended anti-platelet and/or anticoagulant therapy (SFA). 2) Women who are
breastfeeding, pregnant or are intending to become pregnant or men intending to father children
over the next to two years.
Catalogs
No catalogs are available for this product.
See all of Bard Medical‘s catalogsRelated Searches
- Catheter
- Balloon catheter
- Stent
- Dilatation catheter
- Cardiac catheter
- Peripheral catheter
- Metal stent
- Catheter guidewire
- Diagnostic catheter
- Venous catheter
- Access catheter
- Central venous catheter
- Hydrophilic guidewire
- Blood vessel catheter
- PTA catheter
- Nitinol stent
- Triple-lumen catheter
- Self-expanding stent
- Vascular introducer
- Balloon catheter pump
*Prices are pre-tax. They exclude delivery charges and customs duties and do not include additional charges for installation or activation options. Prices are indicative only and may vary by country, with changes to the cost of raw materials and exchange rates.