- Secondary care
- Cardiology
- Venous stent
- Bard Medical
Venous stent E-LUMINEXX®
Add to favorites
Compare this product
Characteristics
- Type
- venous
Description
Features:
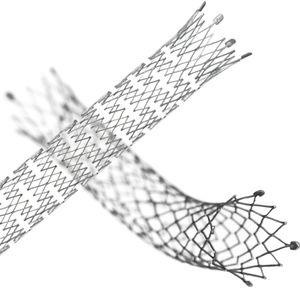
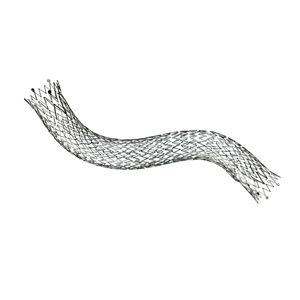
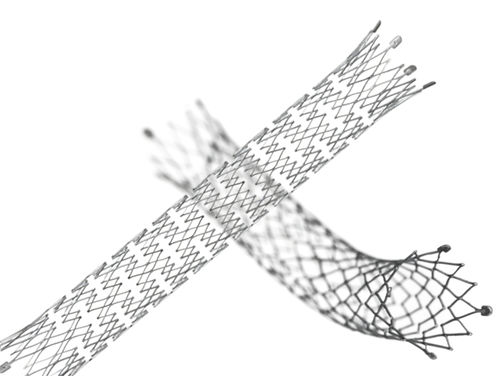
Designed for exceptional visibility under fluoroscopy, which significantly enhances stent placement accuracy
The proprietary interlocking Puzzle® Tantalum marker assures permanent attachment
Dimensional design changes facilitate harmonization of radial force across the range of product diameters
Open cell, flexible mesh design with minimal length change during deployment
2 mm flared stent ends designed to resist migration and compensate for lumen tapering
Read the BARD
® ELUMINEXX
® Vascular Stent IFU thoroughly. Also, thoroughly read the IFUs supplied with any
other interventional devices to be used in conjunction with the system.
• Please use the product illustration at the beginning of this booklet to guide you through the device description.
• Please use the fold-out, step-by-step procedure illustrations at the end of this booklet to guide you through
the procedure description.
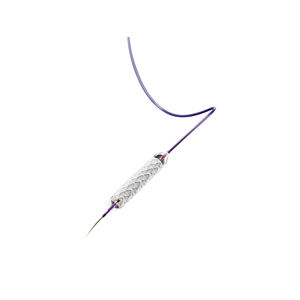
The device is supplied in sterile condition. All materials inside the sterile barrier pouch (the delivery system
and stent as well as the carrier tube and pouchliner) are sterile. The external surface of the sterile pouch and
the product carton should not be considered sterile.
Federal (U.S.A) law restricts this device to sale by or on the order of a physician.
1.0 DEVICE NAME
• The brand name of the device is BARD
® ELUMINEXX
® Vascular Stent.
• The Stent (Implant) is equipped with four highly visible radiopaque PUZZLE
® Tantalum Markers on both the
proximal and distal end
Catalogs
No catalogs are available for this product.
See all of Bard Medical‘s catalogsRelated Searches
- Catheter
- Balloon catheter
- Stent
- Dilatation catheter
- Cardiac catheter
- Peripheral catheter
- Metal stent
- Catheter guidewire
- Diagnostic catheter
- Access catheter
- Venous catheter
- Central venous catheter
- Hydrophilic guidewire
- Blood vessel catheter
- PTA catheter
- Nitinol stent
- Triple-lumen catheter
- Self-expanding stent
- Vascular introducer
- Balloon catheter pump
*Prices are pre-tax. They exclude delivery charges and customs duties and do not include additional charges for installation or activation options. Prices are indicative only and may vary by country, with changes to the cost of raw materials and exchange rates.