- Secondary care
- Cardiology
- Peripheral stent graft
- Bard Medical
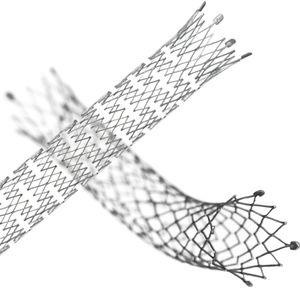
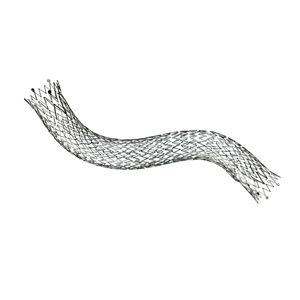
Peripheral stent graft FLUENCY® Plusvenous
Add to favorites
Compare this product
Characteristics
- Type
- peripheral, venous
Description
A broad range of implant diameters and lengths for the treatment of in-stent restenotic peripheral and central lesions* in patients with AV grafts and AV fistulae
Small incremental stent graft lengths to help maintain venous real estate and cannulation area
Minimal shortening and radiopaque markers aid in excellent placement accuracy
Minimal shortening and radiopaque markers
aid in excellent placement accuracy
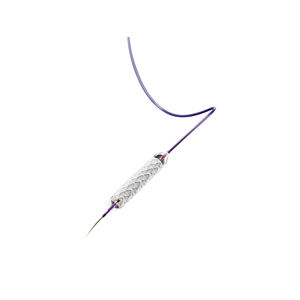
The multi-braided delivery system with a tipless
inner catheter designed to reduce the risk of
catheter entanglement during withdrawal
Dual layer ePTFE encapsulation demonstrated a
significant reduction at 90 days in the incidence
of in-stent restenosis compared to PTA**
Proprietary bioactive carbon impregnation
designed to reduce early stage platelet adhesion
Flexible implant that demonstrated kink
resistance after placement in tortuous AV†
access lesions presenting with ISR†† or nonstented lesions in patients with AV grafts
Prescriptive Information
Prior to use, please see the complete “Instructions for Use” for more information on Indications,
Contraindications, Warnings, Precautions, Adverse Events and Operator’s Instructions.
Indications
The FLUENCY® PLUS Endovascular Stent Graft is indicated for use in the treatment of in-stent restenosis in the
venous outflow of hemodialysis patients dialyzing by either an arteriovenous (AV) fistula or AV graft and for
the treatment of stenosis in the venous outflow of hemodialysis patients dialyzing by an AV graft.
Catalogs
No catalogs are available for this product.
See all of Bard Medical‘s catalogsRelated Searches
- Catheter
- Balloon catheter
- Stent
- Dilatation catheter
- Cardiac catheter
- Peripheral catheter
- Metal stent
- Catheter guidewire
- Diagnostic catheter
- Venous catheter
- Access catheter
- Central venous catheter
- Hydrophilic guidewire
- Blood vessel catheter
- PTA catheter
- Nitinol stent
- Triple-lumen catheter
- Self-expanding stent
- Vascular introducer
- Balloon catheter pump
*Prices are pre-tax. They exclude delivery charges and customs duties and do not include additional charges for installation or activation options. Prices are indicative only and may vary by country, with changes to the cost of raw materials and exchange rates.