- Secondary care
- Cardiology
- Femoral artery stent
- Bard Medical
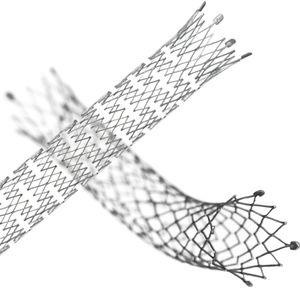
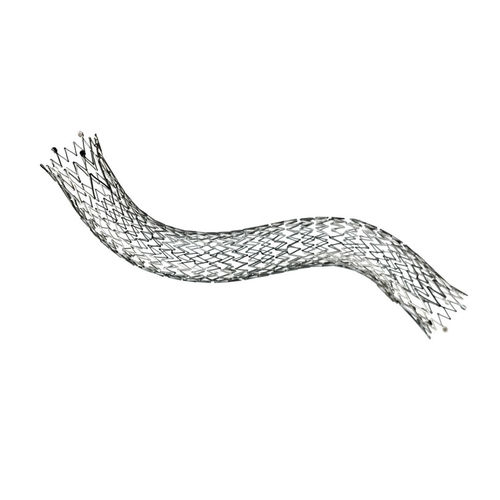
Femoral artery stent LIFESTENT® 5F
Add to favorites
Compare this product
Characteristics
- Type
- femoral artery
Description
Features
Unique helical design engineered for bending, compression, torsion
Only stent FDA-approved for the SFA and full popliteal artery1
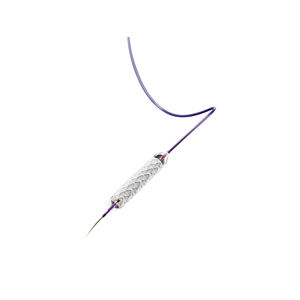
Low profile, 5F delivery system
Dual-speed thumbwheel deployment designed for ease of use and placement accuracy2
GEOALIGN® Marking System designed to increase procedural efficiency and reduce radiation exposure3
Clinically Proven Stent Design:
As the only commercially available stent FDA-approved for the superficial femoral and full popliteal artery, the LIFESTENT® Vascular Stent has a history of proven performance:
In a Level 1 RESILIENT study, the LIFESTENT® Vascular Stent demonstrated treatment superiority over balloon angioplasty with sustained effectiveness out to 3 years4
In the investigator initiated, Level 1 ETAP study of the popliteal artery the LIFESTENT® Vascular Stent demonstrated double the primary patency rate of PTA out to two years5
In a clinical assessment of the treatment of long lesions, the LIFESTENT® Vascular Stent demonstrated high primary patency at 12 months in lesions up to 240mm6
The LIFESTENT® Vascular Stent Systems, in varying sizes, have been studied in more than ten clinical trials globally.Low Profile, 5F Delivery System:
The LIFESTENT® 5F Vascular Stent System offers the same clinically-proven advanced helical stent design as the LIFESTENT® Vascular Stent on a low profile, 5F delivery system. The LIFESTENT® 5F delivery system offers dual speed thumbwheel deployment with a tri-axial catheter that is designed for ease of use, deployment control, and precise placement accuracy
Catalogs
No catalogs are available for this product.
See all of Bard Medical‘s catalogsRelated Searches
- Catheter
- Balloon catheter
- Stent
- Dilatation catheter
- Cardiac catheter
- Peripheral catheter
- Metal stent
- Catheter guidewire
- Diagnostic catheter
- Access catheter
- Venous catheter
- Central venous catheter
- Hydrophilic guidewire
- Blood vessel catheter
- PTA catheter
- Nitinol stent
- Triple-lumen catheter
- Self-expanding stent
- Vascular introducer
- Balloon catheter pump
*Prices are pre-tax. They exclude delivery charges and customs duties and do not include additional charges for installation or activation options. Prices are indicative only and may vary by country, with changes to the cost of raw materials and exchange rates.