- Secondary care
- Cardiology
- Biliary stent
- Bard Medical
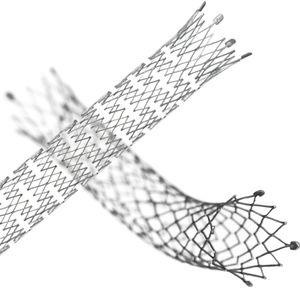
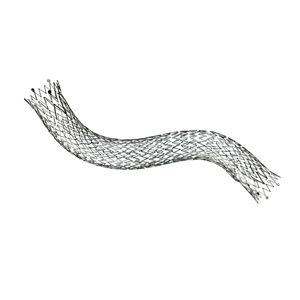
Biliary stent LIFESTENT® and LIFESTENT® XL
Add to favorites
Compare this product
Characteristics
- Type
- biliary
Description
Features:
Longest lesion length indication (up to 240 mm)
Unique helical design
Engineered for bending, compression, torsion
Dynamic vessel conformability
Improved lesion coverage with a single stent
A single-arm, prospective, non-randomized, multi-center study evaluating the safety and
effectiveness of the LIFESTENT® SOLO™ in the treatment of symptomatic vascular disease of the
SFA and/or proximal popliteal artery. Subjects were treated with conventional PTA followed by
implantation of the Bard LIFESTENT® Vascular Stent.
TRIAL OVERVIEW
Q 76 patients
Q 7 study sites in Germany
Q Symptomatic de-novo or restenosed lesions
Q Average lesion length of 91 mm
*The LIFESTENT® Vascular Stent System and the LIFESTENT® SOLO™ Vascular Stent System are intended to improve luminal diameter in the treatment of symptomatic de-novo or
restenotic lesions up to 240 mm in length in the native superficial femoral artery (SFA) and popliteal artery with reference vessel diameters ranging from 4.0-6.5 mm. †
The LIFESTENT® 5 mm diameter was not included in the LIFESTENT® 200 mm Trial.
Catalogs
No catalogs are available for this product.
See all of Bard Medical‘s catalogsRelated Searches
- Catheter
- Balloon catheter
- Stent
- Dilatation catheter
- Cardiac catheter
- Peripheral catheter
- Metal stent
- Catheter guidewire
- Diagnostic catheter
- Access catheter
- Venous catheter
- Central venous catheter
- Hydrophilic guidewire
- Blood vessel catheter
- PTA catheter
- Nitinol stent
- Triple-lumen catheter
- Self-expanding stent
- Vascular introducer
- Balloon catheter pump
*Prices are pre-tax. They exclude delivery charges and customs duties and do not include additional charges for installation or activation options. Prices are indicative only and may vary by country, with changes to the cost of raw materials and exchange rates.