- Secondary care
- Cardiology
- Venous stent
- Bard Medical
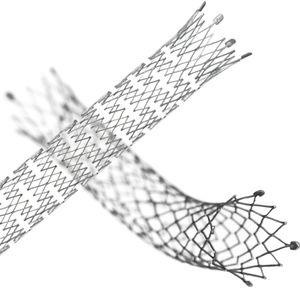
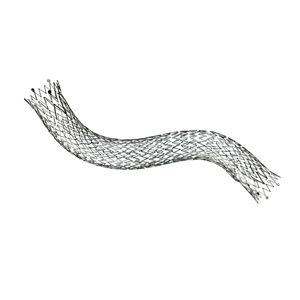
Venous stent VENOVO®
Add to favorites
Compare this product
Characteristics
- Type
- venous
Description
Venovo® Venous Stent System
Purpose-Built for the Iliofemoral Veins
Features:
Designed to provide the optimal balance between radial force, flexibility, and compression resistance
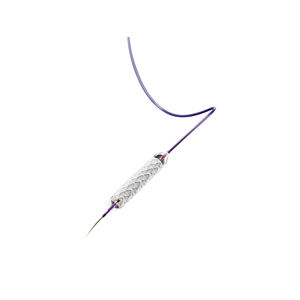
Delivery system designed to provide accurate deployment for optimal stent placement and lesion coverage
Broad range of sizes
Diameters up to 20 mm
Stent lengths up to 160 mm
Clinically proven in Post-Thrombotic and Non-Thrombotic Lesions:
In the VERNACULAR Clinical Study, the VENOVO® Venous Stent demonstrated a primary patency benefit compared to a literature-derived performance goal at 12 months, while demonstrating significant improvement in both VCSS pain scores and quality of life (CIVIQ-20) compared to baseline at 12 months1
88.3% weighted primary patency at 12 months
96.9% patency in non-thrombotic lesions
81.3% patency in post-thrombotic lesions
Zero stent fractures at 12 months
100% acute technical success at time of index procedure
Designed specifically for the venous anatomy
The VENOVO® Venous Stent is specifically designed and developed for the iliofemoral veins.
Catalogs
No catalogs are available for this product.
See all of Bard Medical‘s catalogsRelated Searches
- Catheter
- Balloon catheter
- Stent
- Dilatation catheter
- Cardiac catheter
- Peripheral catheter
- Metal stent
- Catheter guidewire
- Diagnostic catheter
- Access catheter
- Venous catheter
- Central venous catheter
- Hydrophilic guidewire
- Blood vessel catheter
- PTA catheter
- Nitinol stent
- Triple-lumen catheter
- Self-expanding stent
- Vascular introducer
- Balloon catheter pump
*Prices are pre-tax. They exclude delivery charges and customs duties and do not include additional charges for installation or activation options. Prices are indicative only and may vary by country, with changes to the cost of raw materials and exchange rates.