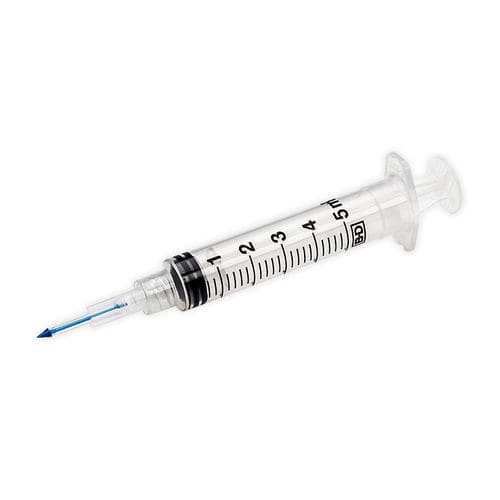
3 mL syringe 303401plasticneedleless
Add to favorites
Compare this product
Characteristics
- Volume
- 3 mL
- Material
- plastic
- Options
- needleless
Description
They are not compatible with conventional injection sites that are not preslit. The BD® Blunt Plastic Cannula is intended for use with Abbott LifeShield®, McGaw SafeLine™ and Baxter InterLink® Systems.
The BD Twinpak™ Dual Cannula Device contains the components you need for syringe filling and needleless delivery of syringe contents into a preslit injection site. The BD Twinpak™ Dual Cannula Device contains an opaque red hub syringe filling device for syringe filling from vials or ampoules and a BD® Blunt Plastic Cannula for safe IV fluid delivery or aspiration through preslit injection sites. The red hub syringe filling device is not intended for accessing injection sites or for percutaneous injections.
BD® Vial Access Cannula are specifically designed for use with the Interlink® injection sites, identified by a colored alert ring around the septum.
Specification
FDA, Premarket Approval, and Regulatory
DEHP is not part of the Material Formulation Yes
Natural Latex No
Packaging
Case Dimensions 16.3in x 14.9in x 13.0in
Quantity 800
Unit of Measure Per case
Product Basic Specification
Weight 11.9 lbs
Manifold None
Sterility Content sterile
Stopcock None
Color of Tubing None
T-Connector Type None
Number of Y-Sites 0
Filter None
Y-Site Port Type None
Number of Backcheck Valves 0
Number of Connectors 0
Number of Fuses 0
Type of Connector None
Catalogs
No catalogs are available for this product.
See all of BD‘s catalogs*Prices are pre-tax. They exclude delivery charges and customs duties and do not include additional charges for installation or activation options. Prices are indicative only and may vary by country, with changes to the cost of raw materials and exchange rates.