- Laboratory
- Laboratory medicine
- Fertility test kit
- Beijing O&D Biotech Co., Ltd.

- Company
- Products
- Catalogs
- News & Trends
- Exhibitions
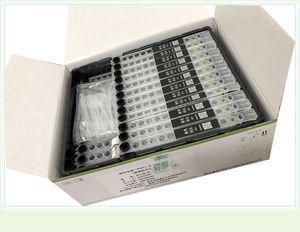
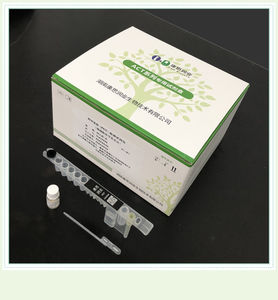
Fertility test kit 0015HCprogesteroneserumCLIA
Add to favorites
Compare this product
Characteristics
- Application field
- fertility
- Tested parameter
- progesterone
- Sample type
- serum
- Analysis mode
- CLIA
- Format
- strip
- Other characteristics
- digital
Description
Product Name: Progesterone Detection Ki(t Magnetic Solid Phase Chemiluminescent Immunoassay)
Packaging: 24 T/KIT
Intended Use:
This product is used for the in vitro quantitative determination of progesterone (P) in human serum. Progesterone is the first biologically active compound produced during steroid biosynthesis and is produced by the adrenal cortex, male testes, female ovaries and placenta. As LH reaches its peak in the middle of the menstrual cycle, the corpus luteum begins to develop and progesterone levels increase. The highest levels are reached 5-8 days after ovulation. If conception does not occur, it decreases to follicular levels about 4 days before the next menstrual period. If pregnancy occurs, progesterone levels continue to increase with the development of the placenta, its main source, and continue throughout pregnancy. Progesterone is mainly used clinically to confirm ovulation, evaluate luteal deficiency, and test the effectiveness of the ovulation induction process. It is now considered that the combined progesterone test is an alternative to endometrial biopsy for the proper evaluation of luteal function, and can even replace endometrial biopsy. Progesterone levels are elevated in both normal and ectopic pregnancies. The measurement of serum progesterone can also be used to monitor the efficacy of replacement therapy and to assess the risk of spontaneous abortion in early pregnancy.
Catalogs
No catalogs are available for this product.
See all of Beijing O&D Biotech Co., Ltd.‘s catalogsOther Beijing O&D Biotech Co., Ltd. products
Chemiluminescence Immunoassay (CLIA) Test Kit
Related Searches
- Assay kit
- Blood assay kit
- Serum assay kit
- Immunoassay assay kit
- Plasma assay kit
- Infectious disease detection kit
- Rapid lateral flow test
- Cassette rapid diagnostic test
- Whole blood detection kit
- Respiratory infection test kit
- Rapid virus test
- Cassette assay kit
- Lateral flow test kit
- COVID-19 assay kit
- Rapid respiratory infection test
- Antigen assay kit
- Strip detection kit
- COVID-19 rapid diagnostic test
- Urine assay kit
- Nasopharyngeal assay kit
*Prices are pre-tax. They exclude delivery charges and customs duties and do not include additional charges for installation or activation options. Prices are indicative only and may vary by country, with changes to the cost of raw materials and exchange rates.