- Laboratory
- Laboratory medicine
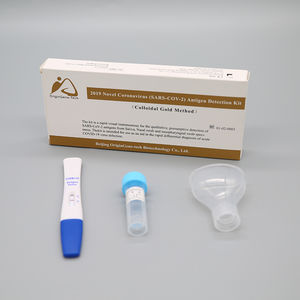
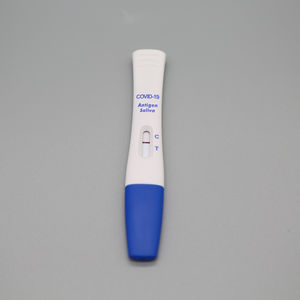
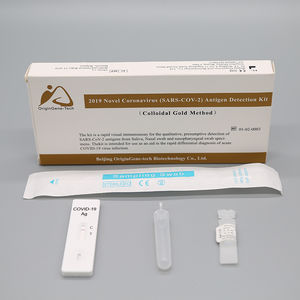
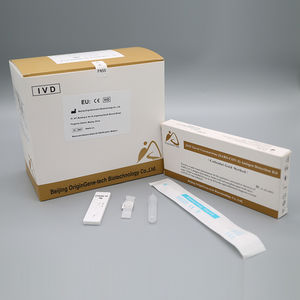
- COVID-19 test kit
- Beijing OriginGene-Tech Biotechnology Co.,Ltd
COVID-19 detection kit influenza Ainfluenza Badenovirus
Add to favorites
Compare this product
Characteristics
- Applications
- COVID-19
- Micro-organism
- influenza A, influenza B, adenovirus, parainfluenza, mumps virus
- Analysis mode
- fluorescence, for real-time PCR
Description
The spirit of self-improvement, pioneering and enterprising, continuous innovation, contribute to improve the level of Chinese national diagnosis industry。
• Reliable diagnosis
High sensitivity and spedficity
• Multiplex real-time PCR
Détection and identification of ORFlab and N gene specifie for 2019-nCoV
•The endogenous internal control
For monitoring over the processes of specimen collection, RT-PCR ampli-fication, thereby reducing false négative results.
► Sample type
Oropharyngeal Swab
►Gene target(s)
(2019-nCoV)ORF1ab» N gene
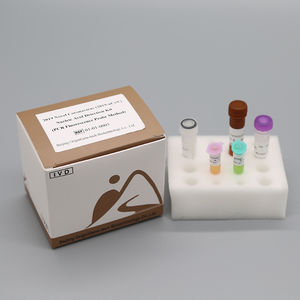
• Number of reactions per kit
24 RXNS
• PCR platform compatibility
Contains FAM, HEXIVIC and Cy5 fluorescence channel
Limit Of Detection (Lod): 250copies/ml
The Kit has no cross reactivity with Influenza A virus H1N1(2009)> H1N1. H3N2* H7N9» H5N1; Influenza B virus Yamagata * Victoria; Human adenovirus 3» 7; Human respiratory syncytial virus; Human parainfluenza virus 2; Haemophilus influenzae; Staphylococcus aureus; Streptococcus pneumoniae; Mumps virus and so on.
Catalogs
No catalogs are available for this product.
See all of Beijing OriginGene-Tech Biotechnology Co.,Ltd‘s catalogsOther Beijing OriginGene-Tech Biotechnology Co.,Ltd products
Diagnostic reagent
Related Searches
- Assay kit
- Solution reagent kit
- Blood assay kit
- Molecular biology reagent kit
- Serum assay kit
- Immunoassay assay kit
- Plasma assay kit
- Infectious disease detection kit
- Blood rapid diagnostic test
- Diagnostic reagent kit
- Rapid lateral flow test
- Laboratory reagent kit
- Molecular test kit
- Whole blood detection kit
- Respiratory infection test kit
- Rapid virus test
- Histology reagent kit
- Optical assay kit
- Reagent medium reagent kit
- Cassette assay kit
*Prices are pre-tax. They exclude delivery charges and customs duties and do not include additional charges for installation or activation options. Prices are indicative only and may vary by country, with changes to the cost of raw materials and exchange rates.