- Laboratory
- Laboratory medicine
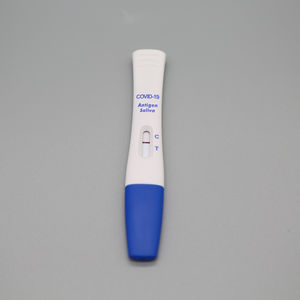
- COVID-19 test kit
- Beijing OriginGene-Tech Biotechnology Co.,Ltd
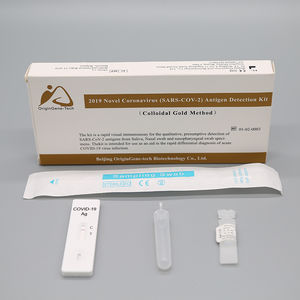
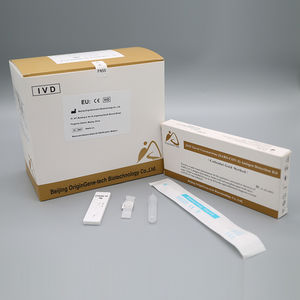
COVID-19 detection kit for antigensSARS-COV-2coronavirus
Add to favorites
Compare this product
Characteristics
- Applications
- COVID-19
- Tested parameter
- for antigens
- Micro-organism
- SARS-COV-2, coronavirus
- Sample type
- nasopharyngeal, nasal
- Analysis mode
- immunoassay
Description
The spirit of self-improvement, pioneering and enterprising, continuous innovation, contribute to improve the level of Chinese national diagnosis industry。
[Intended Use]
The kit is a rapid visual immunoassay for the qualitative, presumptive detection of
SARS-CoV-2 antigens from Nasal swabs and nasopharyngeal swab specimens.
[Feature]
It can quickly detect 2019 Novel Coronavirus (SARS-COV-2) infected or not.
Product Performance
1. The label of the kit is dear and easy to read, and no liquid leakage.
2. Detection limit: Use national reference materials for testing andl meet the national requirements
3. Sample repeatability: Take two repeated reference antigens for testing, and each
reference product is tested 10 times, and the test results are consistent.
4. Reproducibility between batches of reagents: Take three batches of test kits and
test two batches of antigen duplicate reference products respectively. Each reference
product is tested ten times. Ail results were positive for T-line antigen.
5. The SARS-CoV-2 antigen titer of the test product was 1:320, and no hook effect was observed.
Catalogs
No catalogs are available for this product.
See all of Beijing OriginGene-Tech Biotechnology Co.,Ltd‘s catalogsOther Beijing OriginGene-Tech Biotechnology Co.,Ltd products
Diagnostic reagent
Related Searches
- Assay kit
- Solution reagent kit
- Blood assay kit
- Molecular biology reagent kit
- Serum assay kit
- Immunoassay assay kit
- Plasma assay kit
- Infectious disease detection kit
- Blood rapid diagnostic test
- Diagnostic reagent kit
- Laboratory reagent kit
- Rapid lateral flow test
- Molecular test kit
- Rapid virus test
- Respiratory infection test kit
- Whole blood detection kit
- Histology reagent kit
- Optical assay kit
- Reagent medium reagent kit
- Cassette assay kit
*Prices are pre-tax. They exclude delivery charges and customs duties and do not include additional charges for installation or activation options. Prices are indicative only and may vary by country, with changes to the cost of raw materials and exchange rates.