- Laboratory
- Laboratory medicine
- COVID-19 test kit
- Beijing OriginGene-Tech Biotechnology Co.,Ltd
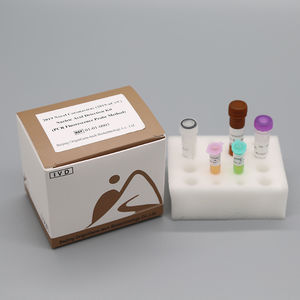
COVID-19 test kit 01-01-0003SARS-COV-2nasopharyngealfor nucleic acids
Add to favorites
Compare this product
Characteristics
- Applications
- COVID-19
- Micro-organism
- SARS-COV-2
- Sample type
- nasopharyngeal, for nucleic acids
- Analysis mode
- for PCR, fluorescence
Description
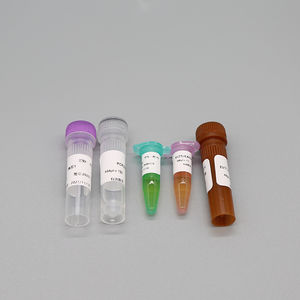
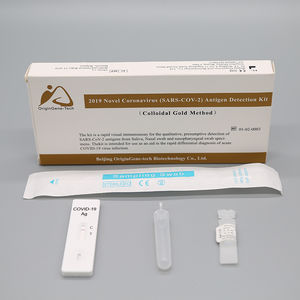
2019 Novel Coronavirus (2019-nCoV) Nucleic Acid Detection Kit (PCR Fluorescence Probe Method) is used for the in vitro qualitative detection of 2019 novel coronavirus (2019-nCoV) RNA in upper respiratory tract specimens (nasopharyngeal and oropharyngeal extracts) by real time PCR systems. It is considered as an aid in the diagnosis of the 2019-nCoV infection.
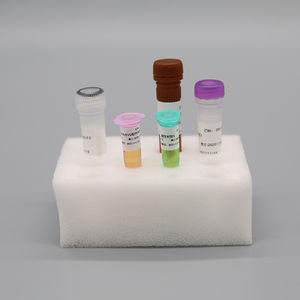
Ref. - Type of Reagent - Presentation 24rxns
1 - PCR Reaction Buffer 1 - 1 vial, 384μL
2 - ORF1ab / N Gene Primer and Probe Mixture - 1 vial, 48μL
3 - Enzyme System 1 - 1 vial, 48μL
4 - Negative Control 1 - 1 vial, 400μL
5 - ORF1ab / N Positive Control - 1 vial, 400μL
Catalogs
Other Beijing OriginGene-Tech Biotechnology Co.,Ltd products
Diagnostic reagent
Related Searches
- Assay kit
- Solution reagent kit
- Blood assay kit
- Molecular biology reagent kit
- Serum assay kit
- Immunoassay assay kit
- Plasma assay kit
- Infectious disease detection kit
- Blood rapid diagnostic test
- Diagnostic reagent kit
- Laboratory reagent kit
- Rapid lateral flow test
- Molecular test kit
- Virus rapid diagnostic test
- Respiratory infection test kit
- Whole blood detection kit
- Histology reagent kit
- Optical assay kit
- Reagent medium reagent kit
- Cassette assay kit
*Prices are pre-tax. They exclude delivery charges and customs duties and do not include additional charges for installation or activation options. Prices are indicative only and may vary by country, with changes to the cost of raw materials and exchange rates.