- Laboratory
- Laboratory medicine
- Staining solution reagent
- BIO-OPTICA Milano
- Company
- Products
- Catalogs
- News & Trends
- Exhibitions
Staining solution reagent Luxol fast blue Kluwer Barrera for cytologyfor histology
Add to favorites
Compare this product
Characteristics
- Type
- staining solution
- Applications
- for histology, for cytology
Description
Minimum number of tests that can be performed 100
Completion time 20 minutes + overnight
Shelf life 2 years
Storage conditions 15-25°C
Additional equipment Not required
Application
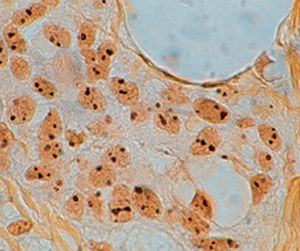
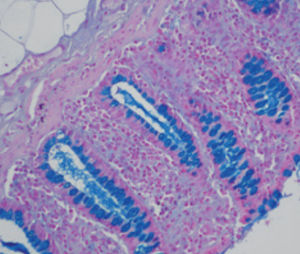
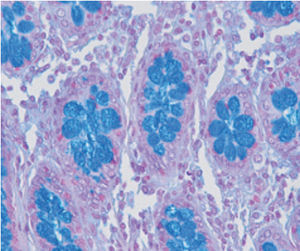
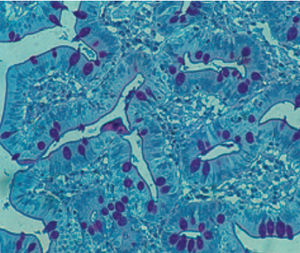
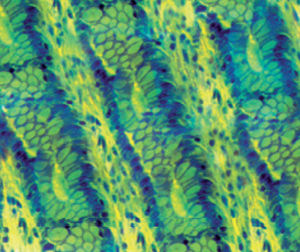
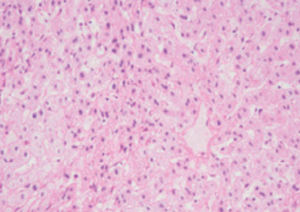
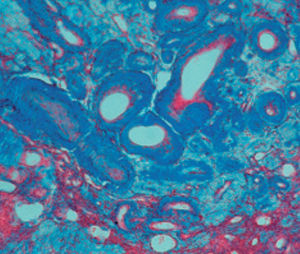
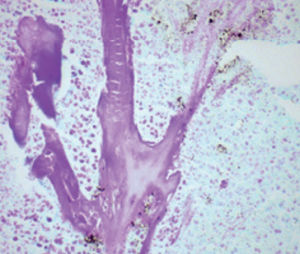
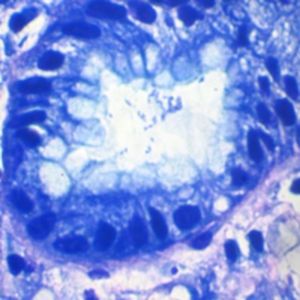
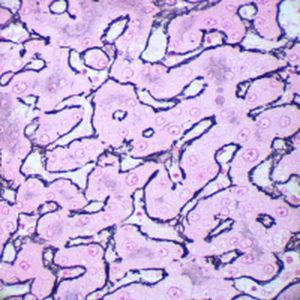
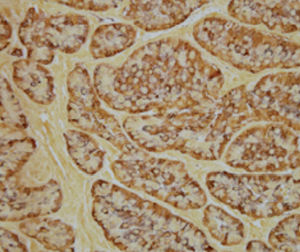
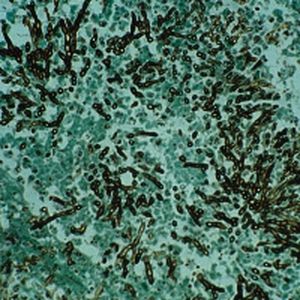
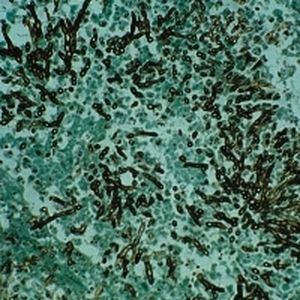
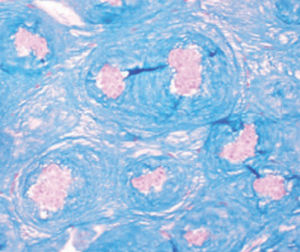
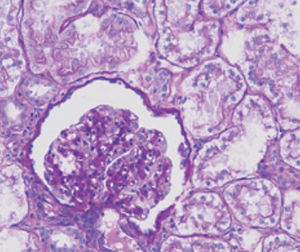
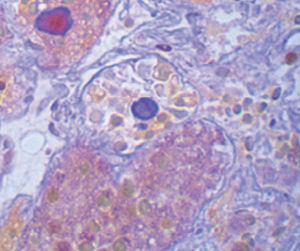
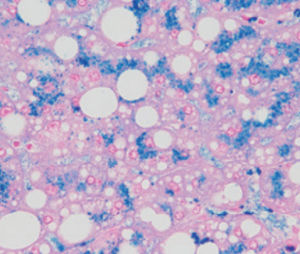
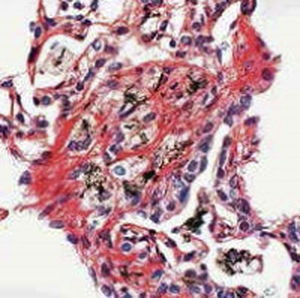
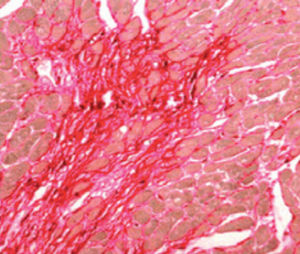
Method indicated for showing myelin and phospholipids on histological sections.
Result
Myelin turquoise blue
Neurons and glial nuclei from pink to violet
Nissl substance pale pink
Product for the preparation of cyto-histological samples for optical microscopy.
To show myelin and phospholipids in histologic sections.
PRINCIPLE
Luxol fast blue dye is a derivative of tetrabenzotetrazo-porphyrin. Kluver has demonstrated porphyrins have a selective affinity for myelin (see references). Luxol fast blue’s affinity for central nervous system is usually ascribed to the bonds it forms with phospholipidic structures such as lecithin and sphingomyelin.
METHOD
1) Deparaffinise and bring section to ethanol 95°.
2) Prepare the incubation box by adding some drops of distilled water on filter paper in Petri dish and lay down the slide; put
on the slide 10 drops of reagent A, close the incubation box and incubate at 56°C overnight in oven.
3) Extract the slide from oven and wash it with ethanol 95° (crystalline residues of reagent A should melt).
4) Wash in distilled water.
5) Put on the section 10 drops of reagent B: leave to act 30 seconds.
6) Differentiate in ethanol 70° until myelinic fibres become blue on colourless background (Sometimes differentiation can be
difficult; repeat the step 5 for 30 seconds and put the slide again in ethanol 70°)
7) Wash well in distilled water (at least 2 times).
Catalogs
General Catalogue
164 Pages
Related Searches
- Bio-Optica solution reagent
- Laboratory reagent kit
- Bio-Optica histology reagent
- Reagent medium reagent kit
- Bio-Optica cytology reagent
- Bio-Optica stain reagent
- Buffer solution reagent kit
- Bacteria reagent kit
- Bio-Optica staining solution reagent
- Microscope slide
- Sample preparation reagent kit
- Pathology reagent
- Bilirubin reagent kit
- Bio-Optica fixative solution reagent
- Paraffin wax reagent
- Microscopy reagent
- Phosphate buffer reagent kit
- Collagen reagent kit
- Helicobacter pylori reagent kit
- Decalcifying solution reagent
*Prices are pre-tax. They exclude delivery charges and customs duties and do not include additional charges for installation or activation options. Prices are indicative only and may vary by country, with changes to the cost of raw materials and exchange rates.