- Laboratory
- Laboratory medicine
- Staining solution reagent
- BIO-OPTICA Milano
- Company
- Products
- Catalogs
- News & Trends
- Exhibitions
Staining solution reagent Perlsfor histologyfor cytology
Add to favorites
Compare this product
Characteristics
- Type
- staining solution
- Applications
- for histology, for cytology
Description
Minimum number of tests that can be performed 72
Completion time 35 minutes
Shelf life 2 years
Storage conditions 15-25°C
Additional equipment 50 ml vertical histology jar, graduated cylinder and glass rod
Application
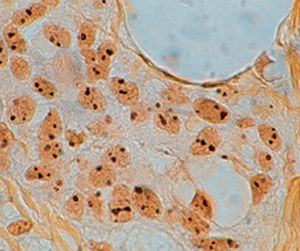
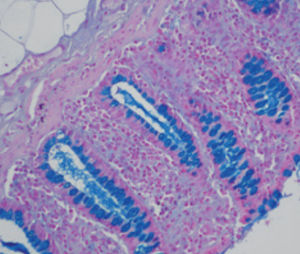
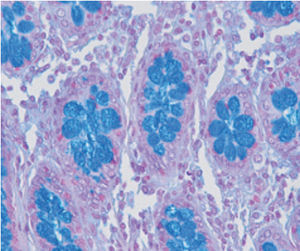
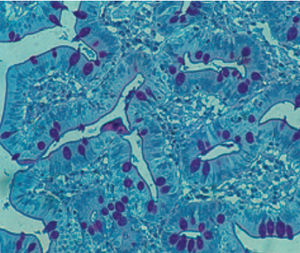
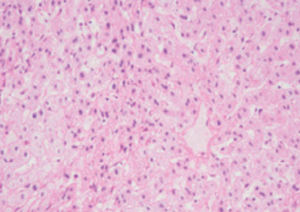
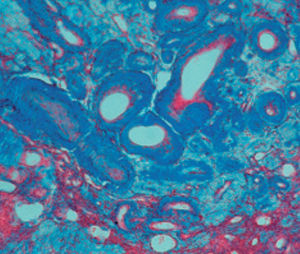
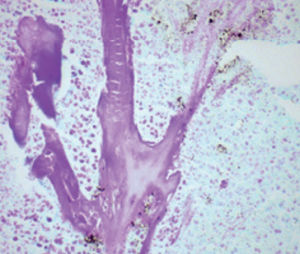
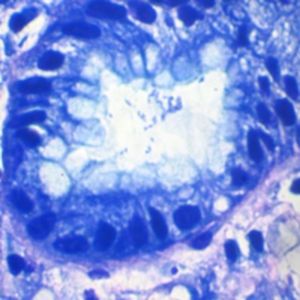
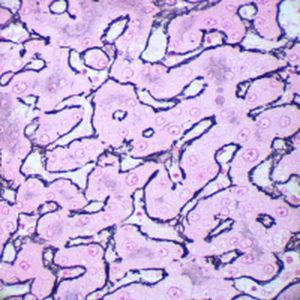
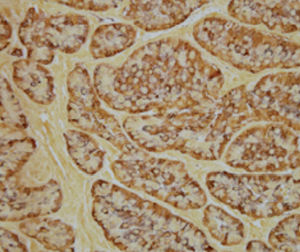
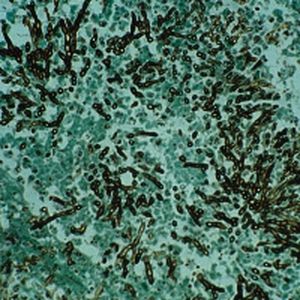
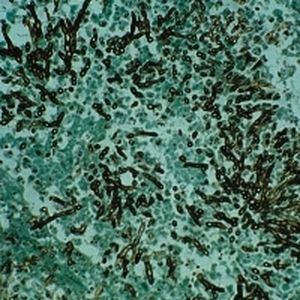
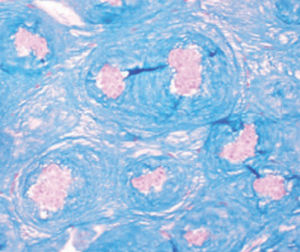
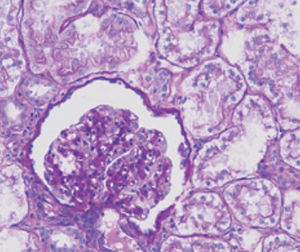
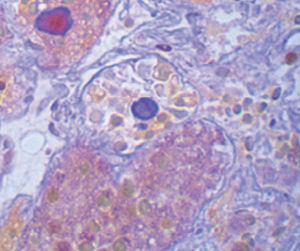
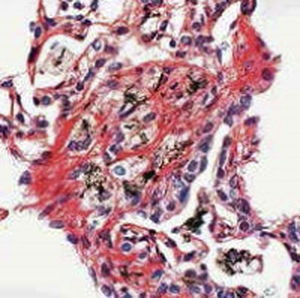
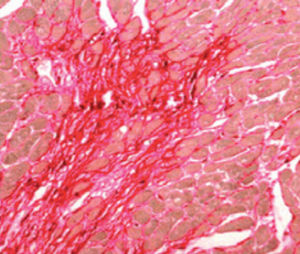
Method indicated for viewing reactive ferric iron on tissue sections, blood smears and
bone marrow smears.
Specificity - the Perls reaction does not show all the iron present in the tissue: iron bound
to hemoglobin, malaria pigment, ferritin, pigments deriving from the use of acid formalin
and ferrous iron does not react.
Result
Reactive ferric iron blue
Nuclei red
Product for the préparation of cyto-histological samples for optical microscopy.
To show reactive ferrie iron in tissue sections (method 1) and for haematology and cytology (method 2).
PRINCIPLE
Potassium ferrocyanide reacts with ferrie ions of hemosiderin in acid environment to form a coloured sait: Prussian blue. Reaction takes place in ionic form as follows: 4 Fe~~ 3K4Fe(CN)6 = Fe4(Fe(CN)6h+ 12 K*
Catalogs
General Catalogue
164 Pages
Related Searches
- Bio-Optica solution reagent
- Laboratory reagent kit
- Bio-Optica histology reagent
- Reagent medium reagent kit
- Bio-Optica cytology reagent
- Bio-Optica stain reagent
- Buffer solution reagent kit
- Bacteria reagent kit
- Bio-Optica staining solution reagent
- Microscope slide
- Sample preparation reagent kit
- Pathology reagent
- Bilirubin reagent kit
- Bio-Optica fixative solution reagent
- Paraffin wax reagent
- Phosphate buffer reagent kit
- Microscopy reagent
- Collagen reagent kit
- Helicobacter pylori reagent kit
- Decalcifying solution reagent
*Prices are pre-tax. They exclude delivery charges and customs duties and do not include additional charges for installation or activation options. Prices are indicative only and may vary by country, with changes to the cost of raw materials and exchange rates.