- Secondary care
- Orthopedic surgery
- Revision knee prosthesis
- Biotech Medical
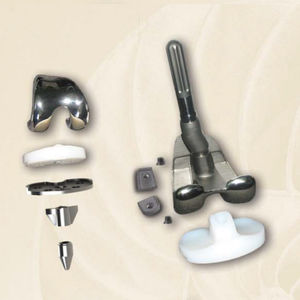
Total knee prosthesis revisionfixed or mobile-bearingcemented or non-cemented
Add to favorites
Compare this product
Characteristics
- Type
- total
- Surgical application
- revision
- Tibial bearing
- fixed or mobile-bearing
- Fixation
- cemented or non-cemented
- Flexion angle
Min.: 5 °
Max.: 9 °
Description
In the Biotech FUTURE KNEE 1 System, there is a wide range of choice of implants meeting the needs of surgeons, which provide a high intraoperative flexibility and minimize the restricitions, defined in the system condition of the case study. According to these, the Biotech FUTURE KNEE 1 System have been designed to provide solutions for Primary, Posterior-Stabilized and Révision indications. The cemented and the uncemented fixation can be flexibly chosen in given intraoperative situations, and can be made with the same set of instruments. The anatomical design of each component allows physiological movements in great extent, overcoming the arising destructive conditions. With total intraoperative flexibility and single, but accurate set of instruments, a natural joint formation can be developed and a long-life expectancy of the artifical joint can be influenced positively.
Important parameters:
•Anatomical CoCr fémoral components
•optional: also available in uncemented version
•CoCr Tibial Tray to minimize polyethylene wear, one piece and modular
•optional: also available in uncemented version and "Ail Poly" tibia
•Primary Tibial Insert lipped
•P/S Fémoral components
•P/S Tibial Insert
•Meniscal bearing components
•Insert screw fixation to reduce micro motion between the insert and the tibial tray
•Revision CoCr femoral components
•Different stem lenghts to increase the joint stability in révision components
•Femoral and Tibial Augmentation Block
•Flexible angular fit (5°, 7°, 9°) of the femoral stems for révision components
•Maximal surface contact of tibial and fémoral components to minimize polyethylene wear
Catalogs
No catalogs are available for this product.
See all of Biotech Medical‘s catalogsRelated Searches
- Bone plate
- Compression plate
- Metallic compression plate
- Locking compression plate
- Distal compression plate
- Compression bone screw
- Metallic compression bone screw
- Interbody fusion cage
- Proximal compression plate
- Forearm compression plate
- Mid-shaft compression plate
- Lateral compression plate
- General purpose compression bone screw
- Tibia compression plate
- Radius compression plate
- PEEK interbody fusion cage
- Femoral stem
- Humeral compression plate
- External fixation system
- Anterior interbody fusion cage
*Prices are pre-tax. They exclude delivery charges and customs duties and do not include additional charges for installation or activation options. Prices are indicative only and may vary by country, with changes to the cost of raw materials and exchange rates.