- Products
- Catalogs
- News & Trends
- Exhibitions
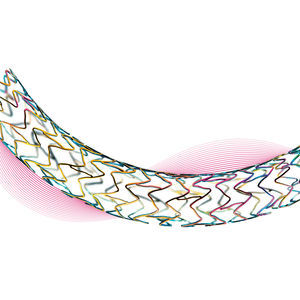
Iliac artery stent Astron®nitinol
Add to favorites
Compare this product
Characteristics
- Type
- iliac artery
- Material
- nitinol
- Diameter
7 mm, 8 mm, 9 mm, 10 mm
(0.276 in, 0.315 in, 0.354 in, 0.394 in)- Length
30 mm, 40 mm, 60 mm, 80 mm
(1.181 in, 1.575 in, 2.362 in, 3.15 in)
Description
Indicated for use in patients with atherosclerotic disease of the iliac arteries and for the treatment of insufficient results after Percutaneous Transluminal Angioplasty (PTA), e.g. residual stenosis and dissection.
Product Highlights
Clinically proven stent for the treatment of iliac disease
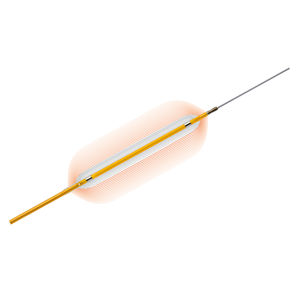
Pull-back delivery system for simple stent deployment
5.2F proximal shaft for contrast injection with device in sheath
Clinically proven stent for the treatment of iliac disease¹
Designed to provide suficient chronic outward force and flexibility
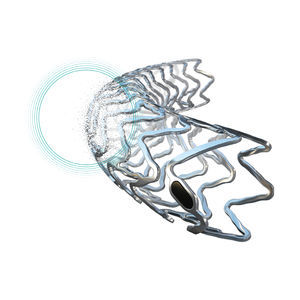
Segmented stent design and strut thickness to provide sufficient chronic outward force in the iliac territory, while the peak-to-valley design and S-articulating connecting bars provide multi-directional flexibility and avoid fish-scaling in tortuous arteries
proBIO® silicon carbide coating reduces ion release by acting as an effective and reliable barrier to nickel and other heavy metal ion diffusion
Pull-back delivery system for simple stent deployment
Pull-back delivery system enables simple stent deployment, while the easy release relieves friction of introducer valve on the retractable shaft during stent deployment, providing a smoother action
5.2F proximal shaft for contrast injection with device in sheath
6F distal shaft with a 5.2F proximal shaft allows contrast injection while the device is positioned inside the introducer and across the lesion
Technical Data
-
Catheter type - OTW
Recommended guide wire - 0.035”
Strut thickness - 225 μm (ø 10 mm = 230 μm)
Stent coating - proBIO® (Amorphous Silicon Carbide)
Stent markers - 4 gold markers each end
Proximal shaft - 5.2F, hydrophobic coating
Catalogs
*Prices are pre-tax. They exclude delivery charges and customs duties and do not include additional charges for installation or activation options. Prices are indicative only and may vary by country, with changes to the cost of raw materials and exchange rates.