- Dental
- Dental practice
- Synthetic bone substitute
- Bonegraft Biomaterials
- Company
- Products
- Catalogs
- News & Trends
- Exhibitions
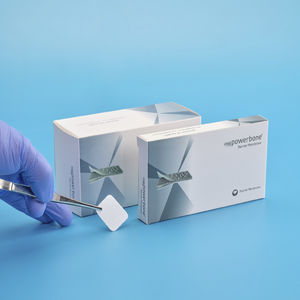
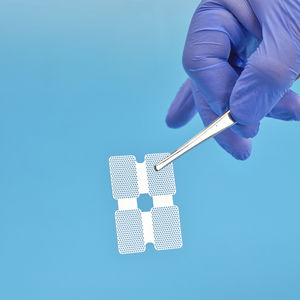
Synthetic bone substitute for dental sugeryfor maxillofacial surgeryrigid
Add to favorites
Compare this product
Characteristics
- Type of graft
- synthetic
- Use
- for dental sugery, for maxillofacial surgery
- Shape
- rigid
Description
The cortical plate consists of synthetic composite materials (PLGA + β-TCP).
Cortical Plate is used for bone fixation in trauma and reconstructive surgical operations.
Cortical Plate is bioabsorbable.
Cortical Plate does not contain human and animal origin tissue or blood derivatives.
Cortical Plate graft is safe for patients undergoing MRI or CT scans and is biocompatible with human tissue.
The shelf life of Cortical Plate is 3 years with sterilisation by ethylene oxide.
Cortical Plate carries a CE mark and is produced in a a ISO 13485 Quality Certified facility.
Catalogs
No catalogs are available for this product.
See all of Bonegraft Biomaterials‘s catalogsOther Bonegraft Biomaterials products
Bonegraft Biomaterials (Membrane and Plate)
Related Searches
- Bone substitute
- Orthopedic surgery bone substitute
- Dental sugery bone substitute
- Synthetic bone substitute
- Granules bone substitute
- Allograft bone substitute
- Rigid bone substitute
- Buccal tissue matrix
- Absorbable tissue matrix
- Putty bone substitute
- Malleable bone substitute
- Powder bone substitute
- Synthetic tissue matrix
- Skull surgery bone substitute
- PLA tissue matrix
*Prices are pre-tax. They exclude delivery charges and customs duties and do not include additional charges for installation or activation options. Prices are indicative only and may vary by country, with changes to the cost of raw materials and exchange rates.