- Laboratory
- Laboratory medicine
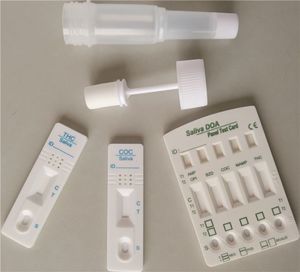
- Rapid drug detection test
- Boson Biotech Co., Ltd.
Rapid drug detection test 1L15S3for tricyclic antidepressantsurineblood
Add to favorites
Compare this product
Characteristics
- Application field
- drug detection
- Tested parameter
- for tricyclic antidepressants
- Sample type
- urine, blood
- Analysis mode
- immunochromatographic
- Format
- cassette, strip
- Result display time
5 min
Description
Rapid Tricyclic antidepressant (TCA) Test is an immunochromatography based one step in vitro test. It is designed for qualitative determination of tricyclic anti-depressant in human urine specimens.
Tricyclic antidepressants, also known now as cyclic antidepressants or TCAs, should be used cautiously in patients with seizures since they can increase the risk of seizures. TCAs may worsen urinary retention and narrow angle glaucoma. Abnormal heart rhythms and sexual dysfunction have also been associated with TCAs. Symptoms of overdose may include: sedation, difficulty thinking, hallucinations, serious changes in heart rhythm, low blood pressure, seizures, hyperthermia, and dilated pupils. TCA can be detected in your urine in 5 days after consumption. Some other medicines can interfere with this test, causing a false-positive for TCAs. These include carbamazepine, quetiapine, diphenhydramine, thioridazine, chlorpromazine, and cyclobenzaprine.
Catalogs
No catalogs are available for this product.
See all of Boson Biotech Co., Ltd.‘s catalogsOther Boson Biotech Co., Ltd. products
Drugs of Abuse Test Kits
Related Searches
- Blood rapid diagnostic test
- Rapid lateral flow test
- Immunoassay rapid diagnostic test
- Cassette rapid diagnostic test
- Virus rapid diagnostic test
- Serum rapid diagnostic test
- Plasma rapid diagnostic test
- Infectious disease rapid diagnostic test
- Whole blood rapid diagnostic test
- Rapid respiratory infection test
- Urine rapid diagnostic test
- Virus reagent kit
- Test strip
- COVID-19 rapid diagnostic test
- Bacteria rapid diagnostic test
- Microbiology reagent kit
- Strip rapid diagnostic test
- Rapid feces test
- Rapid drug abuse test
- Clinical rapid diagnostic test
*Prices are pre-tax. They exclude delivery charges and customs duties and do not include additional charges for installation or activation options. Prices are indicative only and may vary by country, with changes to the cost of raw materials and exchange rates.