- Laboratory
- Laboratory medicine
- Typhoid test kit
- Celnovte Biotechnology Co., Ltd.

- Company
- Products
- Catalogs
- News & Trends
- Exhibitions
Typhoid test kit CF1010for researchfor cancersoncology
Add to favorites
Compare this product
Characteristics
- Applications
- typhoid, for research, for cancers
- Application field
- oncology
- Tested parameter
- for genes, for DNA, for BRAF gene
- Sample type
- clinical, biological, cell, tissue
- Analysis mode
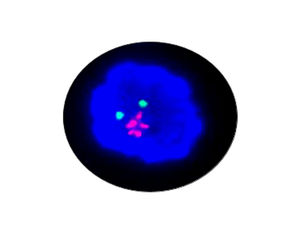
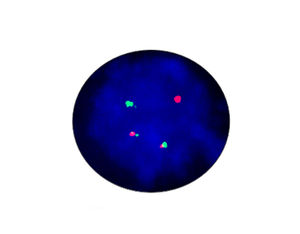
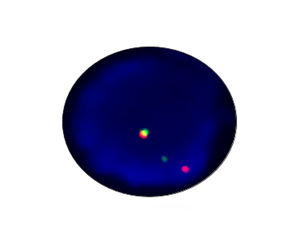
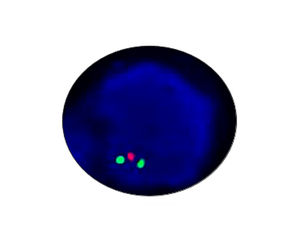
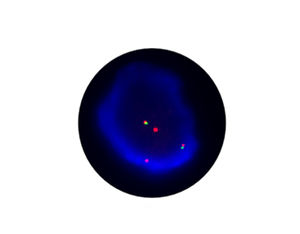
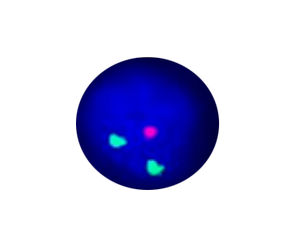
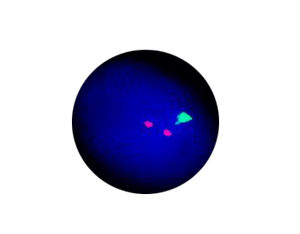
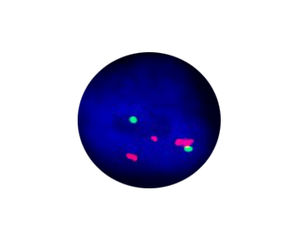
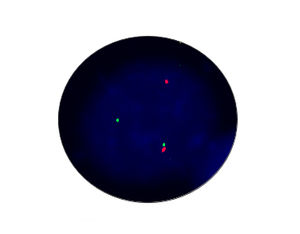
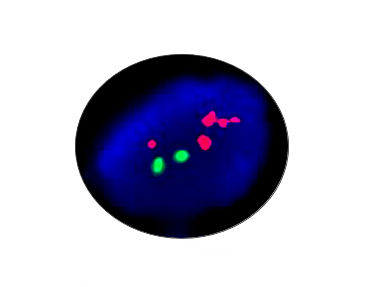
- FISH
- Other characteristics
- custom
Description
1. Guidelines medication:The SFDA has approved Crizotinib as a targeted treatment for advanced ALK-positive non-small cell lung cancer. The essential condition of medicine treatment,XALKORI(Crizotinib), is required to test for ALK-positive non-small cell lung cancer by FISH. At the beginning,IHC preliminary screening could be operated for the sample screening of (D5F3 or 5A4 antibody) and 1+ (more than 5% of the cell color), after then do FISH to confirm positive.ALK gene fusion is an important biological characteristic of non-small cell lung cancer. Patients with positive ALK gene fusion are sensitive to Crizotinib.
2. The monograph, [Chinese Experts Consensus on ALK Positive Non-small cell Lung Cancer Diagnosis (2013 edition illustrates that the proportion of ALK gene positive was as high as 30%-42% in the NSCLC patients group whom is youth (<60 years old) with non-smoking and adenocarcinoma as well as their gene of EGFR, KRAS, HER-2 or P53 has no mutation.
3. Pathological morphology studies suggest that the positive rate in mucus-type or real adenocarcinoma containing imprinted cells is higher than in other types of lung adenocarcinoma.
Catalogs
No catalogs are available for this product.
See all of Celnovte Biotechnology Co., Ltd.‘s catalogsOther Celnovte Biotechnology Co., Ltd. products
FISH
Related Searches
- Assay kit
- Solution reagent kit
- Blood assay kit
- Molecular biology reagent kit
- Immunoassay assay kit
- Infectious disease detection kit
- Research reagent kit
- Protein reagent kit
- Diagnostic reagent kit
- Laboratory reagent kit
- Enzyme reagent kit
- Molecular test kit
- Histology reagent kit
- Clinical assay kit
- Reagent medium reagent kit
- Immunology reagent
- Dye reagent
- Cytology reagent kit
- Antibody
- Buffer solution reagent kit
*Prices are pre-tax. They exclude delivery charges and customs duties and do not include additional charges for installation or activation options. Prices are indicative only and may vary by country, with changes to the cost of raw materials and exchange rates.