- Laboratory
- Laboratory medicine
- COVID-19 test kit
- Changzhou Biowin Pharmaceutical Co., Ltd.
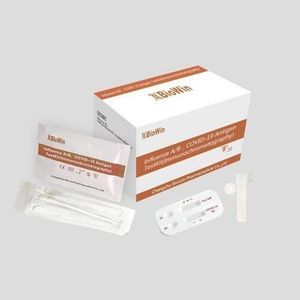
COVID-19 test kit for antigensSARS-COV-2coronavirus

Add to favorites
Compare this product
Characteristics
- Applications
- COVID-19
- Tested parameter
- for antigens
- Micro-organism
- SARS-COV-2, coronavirus
- Sample type
- saliva
- Result display time
15 min
Description
Rapid detection of novel coronavirus.
No instrumentation required: No need for instruments and equipment,suitable for rapid screening.
Suitable for primary screening: Screening for suspected novel coronavirus infected patients.
The Novel Coronavirus (COVID-19) Antigen Test Kit (Colloidal Gold) (For Saliva) is a colloidal gold immunochromatography testing product intended for the qualitative
detection of the nucleocapsid antigens from SARS-CoV-2 in human saliva swabs from individuals who are suspected of COVID-19 within the first seven days of the onset of symptoms.
Results are for the identification of SARS-CoV-2 nucleocapsid antigen. The antigen is generally detectable in upper respiratory samples or lower respiratory samples during the
acutc phase of infection. Positive results indicate the presence of viral antigens, but clinical correlation with patient history and other diagnostic information is necessary to determine
infection status. Positive results do not rule out bacterial infection or co-infection with other viruses.
The test kit detected may not be the definite cause of disease. Negative results do not
rule out SARS-CoV-2 infection and should not be used as the sole basis for treatment or patient management decisions, including infection control decisions. Negative results should be considered in the context of a patient's recent exposures, history and the presence of clinical signs and symptoms consistent with COVID-19
VIDEO
Catalogs
No catalogs are available for this product.
See all of Changzhou Biowin Pharmaceutical Co., Ltd.‘s catalogsOther Changzhou Biowin Pharmaceutical Co., Ltd. products
Covid-19
Related Searches
- Assay kit
- Blood assay kit
- Serum assay kit
- Immunoassay assay kit
- Plasma assay kit
- Infectious disease detection kit
- Rapid virus test
- Respiratory infection test kit
- Whole blood detection kit
- Optical assay kit
- Clinical assay kit
- Fluorescence assay kit
- Lateral flow test kit
- COVID-19 detection kit
- Rapid respiratory infection test
- Real-time PCR test kit
- Antigen assay kit
- IgG test kit
- Strip detection kit
- COVID-19 rapid diagnostic test
*Prices are pre-tax. They exclude delivery charges and customs duties and do not include additional charges for installation or activation options. Prices are indicative only and may vary by country, with changes to the cost of raw materials and exchange rates.