- Laboratory
- Laboratory medicine
- Pneumonia test kit
- Changzhou Biowin Pharmaceutical Co., Ltd.
Pneumonia test kit SARS-COV-2coronavirusnasopharyngeal
Add to favorites
Compare this product
Characteristics
- Applications
- pneumonia
- Micro-organism
- SARS-COV-2, coronavirus
- Sample type
- nasopharyngeal, sputum, throat
- Analysis mode
- for real-time PCR
Description
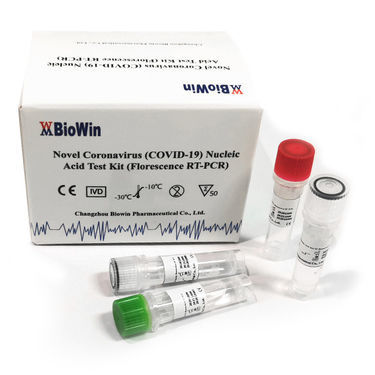
This product is used for the qualitative detection of SARS-CoV-2 (COVID-19) the S and Ngenes in the throat swab and sputum sample. This kit is applicable to qualitatively test of the novel coronavirus(COVID-19,also known as SARS-CoV-2) in a suspected case of pneumonia infected with a Novel Coronavirus,a suspected case of a cluster,other patients who need to be diagnosed with a novel coronavirus infectin or differential diagnosis.
Principle
This kit uses One-Step Real-Time PCR and TaqMan-MGB fluorescent probe technology. Primers and probes are designed in the highly conserved regions of the S and N gene sequences of COVID-19, and human RNase P gene as internal control. This kit has been validated on samples extracted from sputum, nasopharyngeal and throat swabs. Three fluorescent reporters are incorporated into this test: FAM measures the COVID-19 S gene; VIC measures the COVID-19 N gene; CY5 measures human RNase P gene as internal control.
Sample requirements
The nucleic acids extraction in throat swabs or and sputum sample from individual suspected of COVID-19 infection by their health provider is required for the detection. Specimen collection should avoid possible contamination in collection, storage, and transportation. Samples can be inactivated at 56℃ for 30 minutes. Following extraction, RNA samples should be used immediately or stored at -70°C for use later. Avoid and minimize repeated sample freezing and thawing.
Performance Characteristics
Analytical sensitivity (limit of detection) is defined as the lowest concentration at which the detection rate of at least 95% for 20 samples.
Catalogs
No catalogs are available for this product.
See all of Changzhou Biowin Pharmaceutical Co., Ltd.‘s catalogsOther Changzhou Biowin Pharmaceutical Co., Ltd. products
Covid-19
Related Searches
- Assay kit
- Blood assay kit
- Serum assay kit
- Immunoassay assay kit
- Plasma assay kit
- Infectious disease detection kit
- Rapid virus test
- Whole blood detection kit
- Respiratory infection test kit
- Optical assay kit
- Clinical assay kit
- Lateral flow test kit
- Fluorescence assay kit
- COVID-19 assay kit
- Rapid respiratory infection test
- Real-time PCR test kit
- Antigen assay kit
- IgG test kit
- Strip detection kit
- COVID-19 rapid diagnostic test
*Prices are pre-tax. They exclude delivery charges and customs duties and do not include additional charges for installation or activation options. Prices are indicative only and may vary by country, with changes to the cost of raw materials and exchange rates.