- Laboratory
- Laboratory medicine
- Buffer solution reagent
- Charles River Laboratories
- Company
- Products
- Catalogs
- News & Trends
- Exhibitions
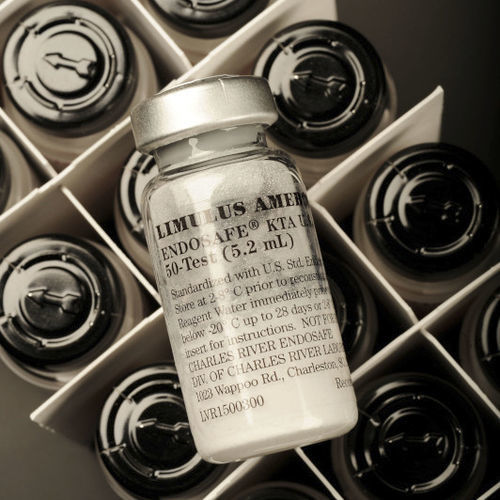
Buffer solution reagent kit LAL for turbidimetrychromogenic
Add to favorites
Compare this product
Characteristics
- Type
- buffer solution
- Applications
- for turbidimetry
- Method
- chromogenic
Description
The kinetic turbidimetric endotoxin test is designed to provide accurate and reliable results on even your most difficult products. Charles River's kinetic turbidimetric assay reagents (KTA and KTA2) allow optimized testing with a single FDA-licensed product that performs both kinetic and gel-clot analysis. With reagents that have faster reaction times and no pre-incubation periods, our KTA2 provides better results and higher throughput than anything else in the industry.
Our two kinetic turbidimetric LAL reagents, KTA and KTA2, yield quantitative endotoxin values when used with a microplate reader equipped with endotoxin-measuring software. Both reagents are buffered to provide significant interference-resistance properties and are FDA-licensed for use in product release testing. Our kinetic turbidimetric assay reagents are buffered to give the best interference-resistance properties.
With KTA and KTA2, you will receive accurate endotoxin results on even your most difficult products in the same amount of time it takes to run a gel-clot LAL test. Our exceptional kinetic turbidimetric endotoxin test formulations are licensed for kinetic and gel-clot analyses and allow for a direct correlation between the methods.
Our traditional kinetic turbidimetric assay reagent (KTA) is suitable for both kinetic and gel-clot analyses and permits a direct correlation between LAL methods.
Catalogs
Other Charles River Laboratories products
Bacterial Endotoxin Testing
Related Searches
- Assay kit
- Solution reagent kit
- Immunoassay assay kit
- Infectious disease detection kit
- Analysis software
- Laboratory reagent kit
- Enzyme reagent kit
- Buffer solution reagent kit
- Control software
- ELISA assay kit
- Bacteria reagent kit
- Laboratory software
- IgG test kit
- Reporting software
- Cell assay kit
- Microbiology reagent kit
- Automated software
- Monitoring software
- Hospital software
- Tracking software
*Prices are pre-tax. They exclude delivery charges and customs duties and do not include additional charges for installation or activation options. Prices are indicative only and may vary by country, with changes to the cost of raw materials and exchange rates.
















