- Laboratory
- Laboratory medicine
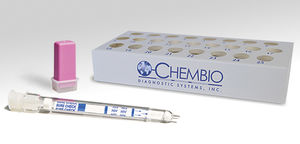
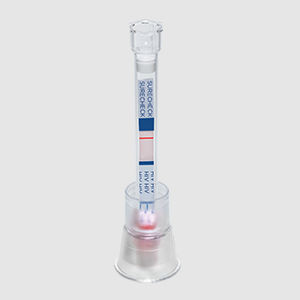
- Rapid syphilis test
- Chembio Diagnostic Systems, Inc.
Rapid syphilis test DPP®HIVTreponema pallidumplasma
Add to favorites
Compare this product
Characteristics
- Applications
- syphilis
- Micro-organism
- HIV, Treponema pallidum
- Sample type
- plasma, whole blood
- Analysis mode
- CLIA
- Other characteristics
- digital
- Result display time
15 min
- Specificity
99.6 %
- Sensitivity
99.4 %
Description
An FDA Approved, CLIA Waived* dual rapid test for the detection of antibodies to HIV 1/2 and Treponema pallidum in fingerstick whole blood, venous whole blood, or plasma specimens.
Reliable
Built-in procedural control
Easy to Use
Provides objective results using simple, handheld digital reader
(DPP® Micro Reader)
Economical
Ideal as the first line rapid test in your algorithm
Are there other confirmatory tests needed when using these rapids?
In the reverse testing algorithm for syphilis, you will need to reflex to RPR to find out if you have an active or past infection. For HIV you would need to confirm a positive based on your site’s protocols.
Could temperature be a factor when using this test in the field or on the street?
The test components should be at room temperature, 18 to 25 C (64 to 77 F), before performing the test and the test should be performed within the same temperature range. If the temperature of the testing area falls outside of 18 to 25 C (64 to 77 F) it is recommended to perform QC prior to testing.
Is this test 4th generation for HIV?
We do not detect antigen to HIV, just antibodies to HIV, the following papers support this approach to the rapid HIV Testing.
The paper ‘Field Accuracy of fourth Generation Rapid Test’ by Lewis et al analyzed 4 studies that included 17,381 samples screened using 4th generation (Antigen-Antibody) rapid tests.
Findings:
26 reference-standard-defined acute HIV infections were missed
35 false positives were observed
Lesson’s Learned: ‘Fourth-generation RDTs are currently unsuitable for the detection of acute HIV-1’ at the point of care.
Catalogs
Other Chembio Diagnostic Systems, Inc. products
Infectious Diseases
Related Searches
- Blood rapid diagnostic test
- Rapid lateral flow test
- Immunoassay rapid diagnostic test
- Cassette rapid diagnostic test
- Rapid virus test
- Serum rapid diagnostic test
- Plasma rapid diagnostic test
- Infectious disease rapid diagnostic test
- Whole blood rapid diagnostic test
- Rapid respiratory infection test
- Bacteria rapid diagnostic test
- POC reader
- Nasal rapid diagnostic test
- Self-test rapid diagnostic test
- Rapid oral flu test
- Laboratory rapid diagnostic test
- Rapid CLIA test
- HIV rapid diagnostic test
- Rapid dengue fever test
- Digital rapid diagnostic test
*Prices are pre-tax. They exclude delivery charges and customs duties and do not include additional charges for installation or activation options. Prices are indicative only and may vary by country, with changes to the cost of raw materials and exchange rates.