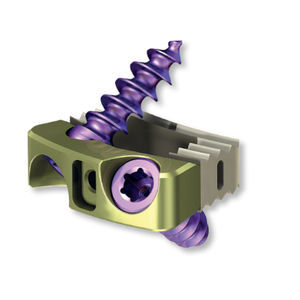
Cervical interbody fusion cage 3.6937.004Santeriortitanium3D-printed
Add to favorites
Compare this product
Characteristics
- Spinal section
- cervical
- Surgical approach
- anterior
- Material
- titanium
- Implant characteristics
- 3D-printed
Description
Cervical intervertebral cage, together with instrument set, is designed for the surgical treatment of the cervical spine diseases at the level of C3 to C7, where spinal arthrodesis is advisable. Cervical spine diseases include:
• hernias,
• Degenerative Disc Diseases (DDD),
• vertebrae instability,
• re-operations,
• degenerative scoliosis.
(The above list is not exhaustive.)
It is not recommended to use the system in case of:
• spine tumors,
• bad physical and mental state of the patient,
• osteoporosis,
• allergy or intolerance to polyetheretherketone (PEEKOptima) or tantalum,
• spine infections,
• vertebral fractures.
(The above list is not exhaustive).
ChM implants have been designed for the best fit to the anatomical shapes of the cervical bodies, to maximize their safety. The arc-shaped anterior wall of the implant imitates the curvature of the anterior part of the vertebral body maximizing the contact surface of the implant with the endplates and eliminating the risk of protruding beyond the line of the bodies. The posterior concavity also ensures the maximum contact surface of the implant with the endplates, minimizing the danger of the pressure being exerted by the cage on the spinal cord. The concave arches of the side walls prevent the vertebral bodies from resting only on the side edges of the cage. Moreover, the cages are offered in a variant with spikes, effectively protecting the cage against migration.
All types and sizes of cervical intervertebral cages differing in size, height and shape of contact surfaces are presented below. All sizes and varieties of cervical intervertebral cages are made of highly biocompatible materials, PEEKand titanium alloy.
Catalogs
No catalogs are available for this product.
See all of ChM‘s catalogsRelated Searches
- Bone plate
- Compression plate
- Metallic compression plate
- Locking compression plate
- Titanium compression plate
- Distal compression plate
- Interbody fusion cage
- Compression bone screw
- Orthopedic surgery surgery set
- Metallic compression bone screw
- Proximal compression plate
- Forearm compression plate
- Mid-shaft compression plate
- Arthrodesis nail
- Lateral compression plate
- Tibia compression plate
- PEEK interbody fusion cage
- Radius compression plate
- Humeral compression plate
- Metallic intramedullary nail
*Prices are pre-tax. They exclude delivery charges and customs duties and do not include additional charges for installation or activation options. Prices are indicative only and may vary by country, with changes to the cost of raw materials and exchange rates.