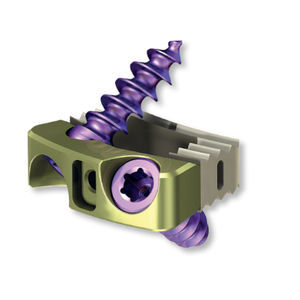
Lumbar interbody fusion cage 3.6925.008Sposteriortitanium3D-printed
Add to favorites
Compare this product
Characteristics
- Spinal section
- lumbar
- Surgical approach
- posterior
- Material
- titanium
- Implant characteristics
- 3D-printed
Description
The PLIF Cage system consists of cages of various widths, heights and lordotic angles to adapt best to variety of patients'anatomies.
PLIF intervertebral cages are made of biocompatible PEEK (polyether ether ketone) polymer, and biocompatible titanium alloy in additive manufacturing technique, by Selective Laser Melting technology.
The implants of PLIF Cage system are designed to be inserted bilaterally (in pairs) and are indicated fora posterior approach for treatment of degenerative disc disease (ODD),vertebral instability, Grade 1 spondylolisthesis as well as for spine revision surgery. The implants should be used at one or two contiguous levels from L2 to Si. DDD is defined as discogenic back pain with degeneration of the disc confirmed by history and radiographic studies. Patients qualified for treatment should be skeletally mature and have had six months of non-operative treatment. The implants of PLIF PEEK Cage system are designed to be used with autogenous bone graft and are intended for use with supplemental fixation systems cleared for use in the lumbar spine (e.g. pedicle screw and rod systems).
The choice of particular device must be carefully considered in terms of patient's overall evaluation.
Circumstances listed below may preclude or reduce the chance of successful outcome:
• Infection, local to the operative site.
• Signs of local inflammation.
• Fever or leukocytosis.
• Morbid obesity {defined according to the W.H.O. standards).
• Pregnancy.
• Neuromuscular disorder which would create unacceptable risk of fixation failure or complications in postoperative care.
Catalogs
No catalogs are available for this product.
See all of ChM‘s catalogsRelated Searches
- Bone plate
- Compression plate
- Metallic compression plate
- Locking compression plate
- Titanium compression plate
- Distal compression plate
- Compression bone screw
- Interbody fusion cage
- Orthopedic surgery surgery set
- Metallic compression bone screw
- Proximal compression plate
- Mid-shaft compression plate
- Forearm compression plate
- Arthrodesis nail
- Lateral compression plate
- Tibia compression plate
- PEEK interbody fusion cage
- Metallic intramedullary nail
- Radius compression plate
- Humerus compression plate
*Prices are pre-tax. They exclude delivery charges and customs duties and do not include additional charges for installation or activation options. Prices are indicative only and may vary by country, with changes to the cost of raw materials and exchange rates.