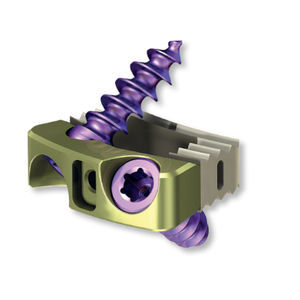
Lumbar vertebral corpectomy prosthesis 15.0914.101expandable
Add to favorites
Compare this product
Characteristics
- Spinal section
- lumbar
- Implant characteristics
- expandable
Description
The CHARSPINE VBR system is intended for reconstruction and stabilization of the thoracolumbar spine for partially or completely removed single-, or multilev- el vertebral bodies. The implants of the CHARSPINE VBR system are designed to replace the removed vertebral bodies by taking over the loads acting on them and to stabilize and maintain the correct curvature of the spine until spondylodesis occurs.
The choice of a particular implant must be carefully considered in terms of patient's medical condition.
Circumstances listed below may preclude or reduce the chance of successful outcome:
Infection, local to the operative site.
Signs of local inflammation.
Fever or leukocytosis.
Morbid obesity {defined according to the W.H.O. standards).
Pregnancy.
Neuromuscular disorder which would create unacceptable risk of fixation failure or complications in postoperative care.
Any other condition which would preclude the potential benefit of spinal implant surgery and disturb the normal process of bone remodeling, e.g. the presence of tumors or congenital abnormalities, fracture local to the operating site, elevation of sedimentation rate unexplained by other diseases.
Suspected or documented allergy or intolerance to implant materials. Where material sensitivity is suspected, appropriate tests should be made prior to material selection or implantation.
Any case not needing a fusion.
Any case not described in the indications.
Any patient unwilling to cooperate with postoperative instructions; mental illness, senility or substance abuse {these conditions may cause the patient to ignore certain necessary limitations and precautions in the use of the implant).
Catalogs
No catalogs are available for this product.
See all of ChM‘s catalogsRelated Searches
- Bone plate
- Compression plate
- Metallic compression plate
- Locking compression plate
- Titanium compression plate
- Distal compression plate
- Compression bone screw
- Metallic compression bone screw
- Interbody fusion cage
- Orthopedic surgery surgery set
- Proximal compression plate
- Forearm compression plate
- Mid-shaft compression plate
- Arthrodesis nail
- Lateral compression plate
- Tibia compression plate
- Radius compression plate
- PEEK interbody fusion cage
- Humeral compression plate
- Metallic intramedullary nail
*Prices are pre-tax. They exclude delivery charges and customs duties and do not include additional charges for installation or activation options. Prices are indicative only and may vary by country, with changes to the cost of raw materials and exchange rates.