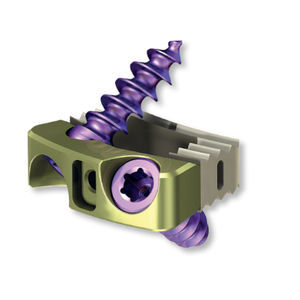
Lumbar interbody fusion cage 40.6125.000transforaminaltitanium
Add to favorites
Compare this product
Characteristics
- Spinal section
- lumbar
- Surgical approach
- transforaminal
- Material
- titanium
Description
TUF Intervertebral cage system is intended for the treatment of diseases caused by disorders of the function of the intervertebral discs in the lumbar spine, at levels from L2 to SI, in skeletally mature patients.The diseases include, i.a.: degenerative disc disease (ODD), vertebral instability, spondylolisthesis, herniated discs. TUF Intervertebral cage system comprises cages of various widths, heights and lordotic angles to adapt best to a patient's spinal anatomy. TUF intervertebral cages are made of biocompatible PEEK {polyether ether ketone) polymer or biocompatible titanium alloy. For the manufacture of the latter, the additive manufacturing technique with use of Selective Laser Melting {SLM) technology {3Dprinting) is used. This technology ensures a spatial structure inside the implant for the bone tissue to overgrow the cage.
The selection of an appropriate device must be preceded by careful and thorough assessment of a patient's state of health.
The conditions listed below may preclude or diminish the chances of successful surgery outcome:
Local infection (at the operative site).
Symptoms of local inflammation.
Fever or high leukocytosis.
Morbid obesity (specified according to the WHO standards).
Pregnancy.
Neuromuscular disorders which could pose a high risk of surgery failure
or occurence of postoperative complications.
Any other condition which could preclude any potential benefits resulting from spinal implant usage and could disturb normal bone remodelling, e.g.: the presence of tumors or congential abnormalities, fracture at the operative site, increase in erythrocyte sedimentation rate unjustified by other diseases.
Catalogs
No catalogs are available for this product.
See all of ChM‘s catalogsRelated Searches
- Bone plate
- Compression plate
- Metallic compression plate
- Locking compression plate
- Titanium compression plate
- Distal compression plate
- Compression bone screw
- Metallic compression bone screw
- Interbody fusion cage
- Orthopedic surgery surgery set
- Proximal compression plate
- Forearm compression plate
- Mid-shaft compression plate
- Arthrodesis nail
- Lateral compression plate
- Tibia compression plate
- Radius compression plate
- PEEK interbody fusion cage
- Humeral compression plate
- Metallic intramedullary nail
*Prices are pre-tax. They exclude delivery charges and customs duties and do not include additional charges for installation or activation options. Prices are indicative only and may vary by country, with changes to the cost of raw materials and exchange rates.