- Secondary care
- Orthopedic surgery
- Cervical vertebra arthrodesis plate
- Chongqing FWS Medical Devices
- Products
- Catalogs
- News & Trends
- Exhibitions
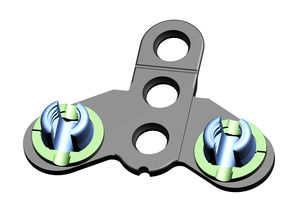
Cervical vertebra arthrodesis plate JQL seriestitanium1 level2 levels
Add to favorites
Compare this product
Characteristics
- Applications
- cervical vertebra
- Materials
- titanium
- Number of fused vertebrae
- 2 levels, 1 level, 3 levels
Description
The fixed titanium plate is made of TA3G pure titanium material conforming to the GB/T13810 standard, and the screw is made of TC4 titanium alloy material conforming to the GB/T13810 standard. The surface of the fixed titanium plate and the screw is treated with colored anodizing. Non-sterile packaging
Instructions
The implantation of the device should be performed by a physician who fully understands the device, its use, equipment and the required surgical techniques
Features
Large bone graft window to provide better surgical vision
Multiple fixation modes, providing strong fixation semi-rigid fixation and mixed fixation modes to meet different cliniacl needs
Cam locking design, locking is accurate and reliable, providing multi-sensory locking confirmation
Low notch, allenviate dysphaga after operation
Scope of application
It is suitable for cervical spine fracture, vertebral body resection, degenerative disease, anatomical variation, and dislocation surgery for anterior cervical internal fixation. The product should be used with intervertebral fusion cage when used for vertebral body resection surgery.
Other Chongqing FWS Medical Devices products
Cervical Anterior Fixator System
Related Searches
- Bone plate
- Compression plate
- Metallic compression plate
- Locking compression plate
- Titanium compression plate
- Distal compression plate
- Interbody fusion cage
- Compression bone screw
- Orthopedic surgery surgery set
- Metallic compression bone screw
- Proximal compression plate
- Forearm compression plate
- Mid-shaft compression plate
- Arthrodesis nail
- Lateral compression plate
- Tibia compression plate
- PEEK interbody fusion cage
- Radius compression plate
- General purpose compression bone screw
- Humeral compression plate
*Prices are pre-tax. They exclude delivery charges and customs duties and do not include additional charges for installation or activation options. Prices are indicative only and may vary by country, with changes to the cost of raw materials and exchange rates.