- Secondary care
- Cardiology
- Aortic valve bioprosthesis
- Colibri Heart Valve

- Products
- Catalogs
- News & Trends
- Exhibitions
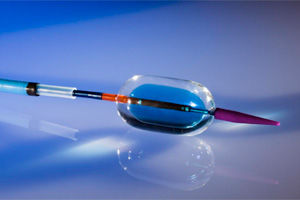
Aortic valve bioprosthesis Colibriporcine tissuesutureless
Add to favorites
Compare this product
Characteristics
- Valve type
- aortic
- Material
- porcine tissue
- Features
- sutureless
Description
Colibri recently initiated a clinical early feasibility study (EFS) which is an international prospective, non-randomized, single arm, open label, trial to assess early feasibility of the Colibri second-generation 24mm and 27mm percutaneous, ready-for-use, balloon expandable aortic heart valve and delivery system. The company’s second-generation TAVI system includes enhancements to address a wider range of patient candidates including those with bicuspid aortic valves and younger patients that need a more durable valve.
*Prices are pre-tax. They exclude delivery charges and customs duties and do not include additional charges for installation or activation options. Prices are indicative only and may vary by country, with changes to the cost of raw materials and exchange rates.