- Laboratory
- Laboratory medicine
- COVID-19 test kit
- Convergent Technologies

- Products
- Catalogs
- News & Trends
- Exhibitions
COVID-19 test kit Convergys®geneticvirusSARS-COV-2
Add to favorites
Compare this product
Characteristics
- Applications
- COVID-19
- Tested parameter
- genetic
- Micro-organism
- virus, SARS-COV-2, coronavirus, influenza A, influenza B
- Sample type
- clinical
- Analysis mode
- real-time
- Result display time
90 min
- Sample volume
200 ml
(6.7628 US fl oz)
Description
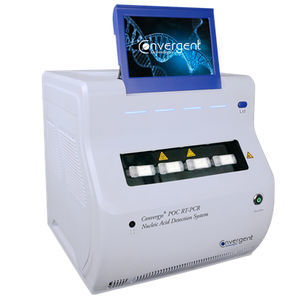
Convergys® POC RT-PCR COVID-19/Influenza A/Influenza B Detection Kit
for use with the Convergys® POC RT-PCR Nucleic Acid Detection System
Infections with SARS-CoV-2 or Influenza virus can cause very similar symptoms, which is a challenge for healthcare professionals.
The Convergys® POC RT-PCR COVID-19/Influenza A/Influenza B Detection Kit directly detects SARS-CoV-2 and Influenza virus A and B genetic material, to assist healthcare professionals in clinical differential diagnosis.
Main Features
Qualitative detection of the novel coronavirus SARS-CoV-2 and Influenza viruses A and B
Real-time PCR methodology
Targets specific nucleotide sequences of SARS-CoV-2 and Influenza viruses A and B
Cartridge based kit for the use with the Convergys® POC RT-PCR system
The Convergys® POC RT-PCR system is fully automated:
Easy operation
Reduced hands-on time
Reduced exposure to infectious material
Reduced risk of application errors
Result within 90 minutes
Internal control material for validation of results
Direct application of sample material, no previous extraction is required
Catalogs
Exhibitions
Meet this supplier at the following exhibition(s):

Related Searches
- Assay kit
- Infectious disease detection kit
- Rapid lateral flow test
- Virus rapid diagnostic test
- Respiratory infection test kit
- Clinical assay kit
- COVID-19 detection kit
- Rapid respiratory infection test
- COVID-19 rapid diagnostic test
- Genetic test kit
- Coronavirus detection kit
- Nasal rapid diagnostic test
- Nasopharyngeal rapid diagnostic test
- Real-time detection kit
- Influenza A assay kit
- Influenza B assay kit
- Nucleocapsid protein rapid test
*Prices are pre-tax. They exclude delivery charges and customs duties and do not include additional charges for installation or activation options. Prices are indicative only and may vary by country, with changes to the cost of raw materials and exchange rates.