- Products
- Catalogs
- News & Trends
- Exhibitions
Thoraco-lumbo-sacral osteosynthesis unit BDYNposterioradult
Add to favorites
Compare this product
Characteristics
- Spinal section
- thoraco-lumbo-sacral
- Surgical approach
- posterior
- Patient type
- adult
Description
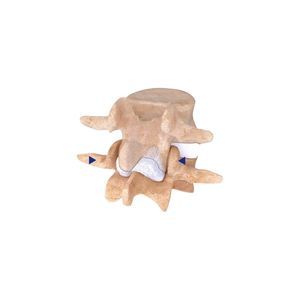
BDyn is a posterior dynamic motion preservation system which is designed for application from thoracic vertebrae T10 to sacrum S1. The dynamic rod BDyn is composed of a silicone cushion, that helps to decrease intradiscal pressure and to relieve facet loads, and an elastomere ring that controls hyper mobility and limits extention of the spine segment. BDyn enables all anatomical motion such as flexion/extension, axial rotation as well as lateral bending. Beside a one level motion preservation device, BDyn has also a version with a longer fusion rod to combine 1 or 2 levels of fusion with motion preservation for the adjacent segment. BDyn is indicated for lombar surgery in case of :
Degenerative intervertebral disc disease
Spinal canal stenosis
Degenerative spondylolisthesis grade 1
Segmental instability
Technical specifications
Legal notice: BDyn is a class IIb medical devices manufactured by COUSIN BIOTECH s.a.s. The CE conformity has been carried out by the notified body SGS Belgium NV (CE1639). The management system of COUSIN BIOTECH S.A.S is certified for compliance with ISO 13485 standard. Please read carefully the instructions for use before using the device. Cousin Biotech is the legal manufacturer of the medical devices proposed by Cousin Surgery.
*Prices are pre-tax. They exclude delivery charges and customs duties and do not include additional charges for installation or activation options. Prices are indicative only and may vary by country, with changes to the cost of raw materials and exchange rates.